null
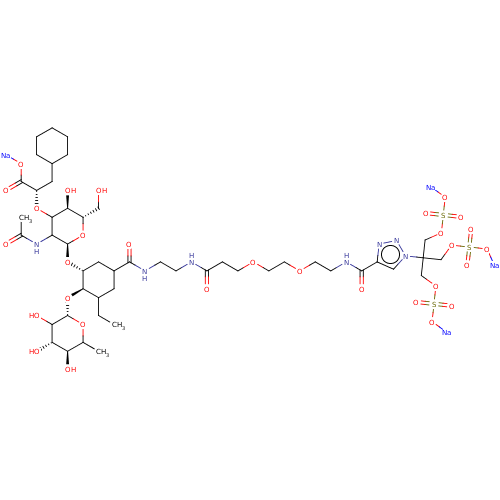
SMILES CCC1CC(C[C@@H](O[C@@H]2O[C@@H](CO)[C@H](O)C(O[C@@H](CC3CCCCC3)C(=O)O[Na])C2NC(C)=O)[C@@H]1O[C@@H]1OC(C)[C@@H](O)[C@H](O)C1O)C(=O)NCCNC(=O)CCOCCOCCNC(=O)c1cn(nn1)C(COS(=O)(=O)O[Na])(COS(=O)(=O)O[Na])COS(=O)(=O)O[Na]
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 528669
Found 1 hit for monomerid = 528669
Affinity DataIC50: 192nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair