null
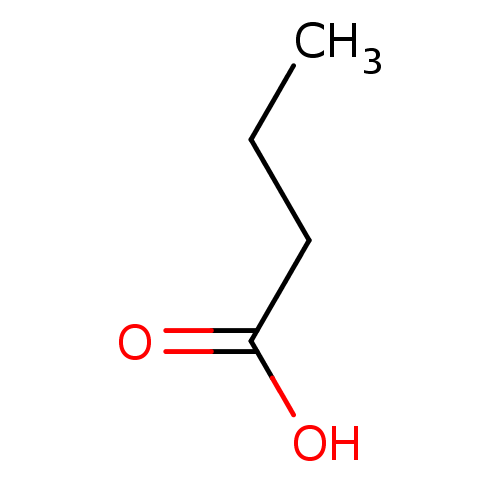
SMILES CCCC(O)=O
InChI Key InChIKey=FERIUCNNQQJTOY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 18 hits for monomerid = 26109
Found 18 hits for monomerid = 26109
Affinity DataKi: 8.20E+4nMAssay Description:Inhibition of mouse Oat6-mediated [3H]ES uptake in Xenopus oocytes after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 1.36E+5nMAssay Description:Inhibition of HDAC in human Hela cells nuclear extracts by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.50E+6nMAssay Description:Inhibition of mouse Oat1-mediated [3H]PAH uptake in Xenopus oocytes after 1 hrMore data for this Ligand-Target Pair
TargetHistone deacetylase 2(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human recombinant HDAC2More data for this Ligand-Target Pair
TargetHistone deacetylase 3(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibition of human recombinant HDAC3More data for this Ligand-Target Pair
TargetHistone deacetylase 8(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of human recombinant HDAC8More data for this Ligand-Target Pair
TargetHistone deacetylase 4(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 2.00E+6nMAssay Description:Inhibition of human recombinant HDAC4More data for this Ligand-Target Pair
TargetHistone deacetylase 5(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 2.00E+6nMAssay Description:Inhibition of human recombinant HDAC5More data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 2.00E+6nMAssay Description:Inhibition of human recombinant HDAC7More data for this Ligand-Target Pair
TargetHistone deacetylase 9(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 2.00E+6nMAssay Description:Inhibition of human recombinant HDAC9More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 2.00E+6nMAssay Description:Inhibition of human recombinant HDAC6More data for this Ligand-Target Pair
TargetFree fatty acid receptor 3(Homo sapiens (Human))
Department of Pharmacy and Biotechnology, University of Bologna , Via Belmeloro 6, 40126 Bologna, Italy.
Curated by ChEMBL
Department of Pharmacy and Biotechnology, University of Bologna , Via Belmeloro 6, 40126 Bologna, Italy.
Curated by ChEMBL
Affinity DataEC50: 1.20E+4nMAssay Description:Agonist activity at human GPCR41 transfected in HEK293 cells assessed as [35S]GTPgammaS binding by scintillation counting methodMore data for this Ligand-Target Pair
TargetHistone deacetylase 2(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibition of human recombinant HDAC1More data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 1.00E+7nMpH: 7.5 T: 2°CAssay Description:A coupled-assay for JMJD2E activity employing formaldehyde dehydrogenase (FDH) from Pseudomonas putida was developed. Formaldehyde release by demethy...More data for this Ligand-Target Pair
TargetMethyl-accepting chemotaxis protein NahY(Pseudomonas putida (Arthrobacter siderocapsulatus))
CSIC
CSIC
Affinity DataKd: 9.20E+4nMT: 2°CAssay Description:Measurements were done on a VP-microcalorimeter (MicroCal, Amherst, MA).More data for this Ligand-Target Pair
Activity Spreadsheet -- ITC Data from BindingDB
 Found 1 hit for monomerid = 26109
Found 1 hit for monomerid = 26109
CellMethyl-accepting chemotaxis protein (McpS)(Pseudomonas putida (Arthrobacter siderocapsulatus))
CSIC
CSIC
ITC DataΔG°: -5.41kcal/mole −TΔS°: 2.80kcal/mole ΔH°: -7.88kcal/mole logk: 1.09E+4
T: 20.00°C
T: 20.00°C
 3D Structure (crystal)
3D Structure (crystal)