null
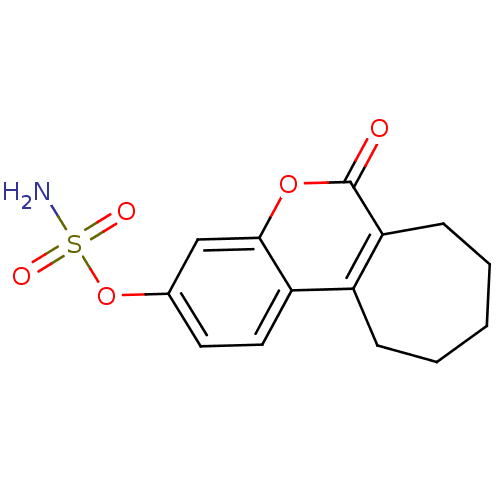
SMILES NS(=O)(=O)Oc1ccc2c3CCCCCc3c(=O)oc2c1
InChI Key InChIKey=DSLPMJSGSBLWRE-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 41 hits for monomerid = 13058
Found 41 hits for monomerid = 13058
Affinity DataKi: 12nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 21nM ΔG°: -10.5kcal/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 24nM ΔG°: -10.4kcal/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 34nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 653nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 720nM ΔG°: -8.37kcal/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 755nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 765nM ΔG°: -8.34kcal/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 1.05E+3nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 3.45E+3nM ΔG°: -7.45kcal/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 7.00E+5nM ΔG°: -4.30kcal/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Compound was tested for inhibition of human carbonic anhydrase (hCA II)More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Tested for the inhibitory activity against Estrone SulfataseMore data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Inhibition of estrone sulfataseMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of steroid sulfatase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of steroid sulfatase in human JEG3 cells by scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of aromatse in human JEG3 cells by scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Compound was evaluated for its inhibitory activity against estrone sulfatase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of STS in human T47D cells preincubated for 1 hr followed by addition of [3H]-E1S/E1S as substrate measured after 24 hrs by HPLC based rad...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of human placental microsomal STS assessed as formation of E1 preincubated for 30 mins followed by addition of E1S as substrate measured a...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Irreversible inhibition of STS in human T47D cells preincubated for 2 hrs followed by compound washout and addition of [3H]-E1S/E1S as substrate meas...More data for this Ligand-Target Pair
Affinity DataIC50: 6.40nMAssay Description:Inhibition of steroid sulfatase in human JEG3 cell lysates using [3H] E1S as substrate after 1 hr by scintillation spectrometry analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of steroid sulfatase in human JEG3 cells using [3H] E1S as substrate incubated for 20 hrs by scintillation spectrometry analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Irreversible inhibition of steroid sulfatase in human intact MCF7 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibition of steroid sulfatase in human intact MCF7 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of placental microsomal estrone sulfatase (unknown origin) using [3H]-estrone sulphate as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 421nMAssay Description:Inhibition of steroid sulfatase in human JEG-3 cells assessed as [14C]-Estrone formation using [3H]E1S as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of Estrone sulfatase in human placental microsomeMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMpH: 7.8 T: 2°CAssay Description:The in vitro inhibition of carbonic anhydrase was assessed by a colorimetric assay. Carbonic anhydrase-catalysed hydrolysis of p-nitrophenyl acetate ...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibitory activity to dihydrofolate reductase in Escherichia coliMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 22nMpH: 7.8 T: 2°CAssay Description:The in vitro inhibition of carbonic anhydrase was assessed by a colorimetric assay. Carbonic anhydrase-catalysed hydrolysis of p-nitrophenyl acetate ...More data for this Ligand-Target Pair
Affinity DataKd: 45nMpH: 7.6 T: 2°CAssay Description:Dissociation constant of 667-coumate was measured for tight-binding inhibitors of CA II. The formation of the 4-nitrophenol was monitored at 348 nm f...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)