null
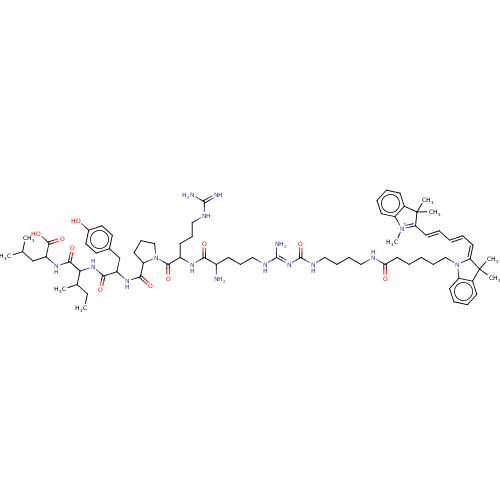
SMILES [Br-].OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C1CCCN1C(=O)C(CCCNC(N)=N)NC(=O)C(N)CCCN\C(N)=N\C(=O)NCCCCNC(=O)CCCCCN1\C(=C/C=C/C=C/C2=[N+](C)c3ccccc3C2(C)C)C(C)(C)c2ccccc12)C(=O)NC(CC(C)C)C(O)=O
InChI Key InChIKey=SBBBJIHYZUEKOH-NHUHVMOSSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50159186
Found 2 hits for monomerid = 50159186
Affinity DataKi: 4.10nMAssay Description:Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29...More data for this Ligand-Target Pair
Affinity DataKi: 7.90nMAssay Description:Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from human NTSR1 transfec...More data for this Ligand-Target Pair