null
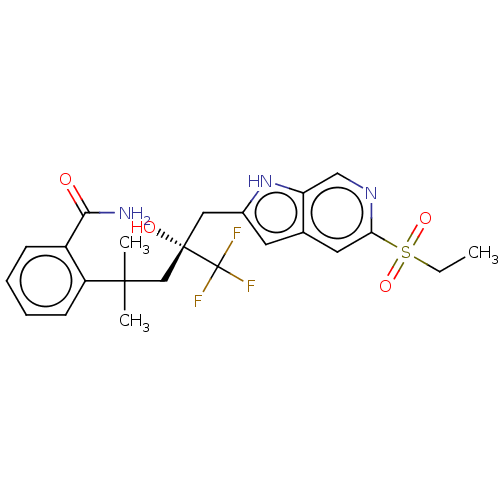
SMILES CCS(=O)(=O)c1cc2cc(C[C@](O)(CC(C)(C)c3ccccc3C(N)=O)C(F)(F)F)[nH]c2cn1
InChI Key InChIKey=HYFZBJIDQLQGIG-QFIPXVFZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50041893
Found 8 hits for monomerid = 50041893
TargetGlucocorticoid receptor(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 43nMAssay Description:Agonist activity at GR in HFF cells assessed as suppression of IL-1-induced IL-6 productionMore data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 95nMAssay Description:Displacement of RU-486 from GR (unknown origin) by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.40E+4nMAssay Description:Inhibition of human recombinant CYP3A4 using 7-benzyloxy-4-(trifluoromethyl)-coumarin as substrate after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG channel expressed in recombinant HEK293 cells by whole cell patch-clamp techniqueMore data for this Ligand-Target Pair