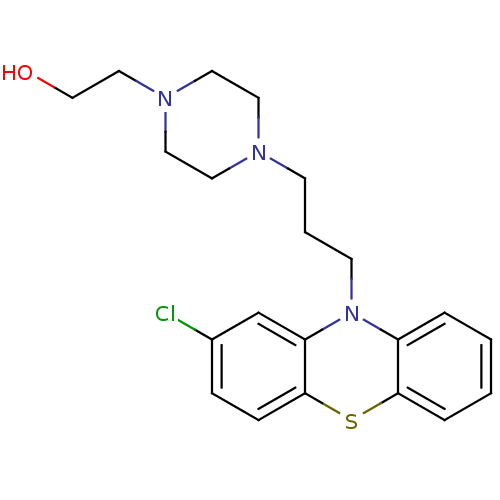
null
SMILES OCCN1CCN(CCCN2c3ccccc3Sc3ccc(Cl)cc23)CC1
InChI Key InChIKey=RGCVKNLCSQQDEP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 68 hits for monomerid = 50130273
Found 68 hits for monomerid = 50130273
TargetD(2) dopamine receptor(Rattus norvegicus (rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
TargetD(2) dopamine receptor(Rattus norvegicus (rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
TargetD(2) dopamine receptor(Rattus norvegicus (rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
TargetHistamine H1 receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetHistamine H1 receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetHistamine H1 receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 6(RAT)
Friedrich-Schiller-University Jena
Curated by PDSP Ki Database
Friedrich-Schiller-University Jena
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 6(Homo sapiens (Human))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 6(RAT)
Friedrich-Schiller-University Jena
Curated by PDSP Ki Database
Friedrich-Schiller-University Jena
Curated by PDSP Ki Database
TargetSigma non-opioid intracellular receptor 1(Cavia porcellus (Guinea pig))
TBA
Curated by PDSP Ki Database
TBA
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 7(Rattus norvegicus (rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
Virginia Commonwealth University
Curated by ChEMBL
Virginia Commonwealth University
Curated by ChEMBL
Affinity DataKi: 23nMAssay Description:Binding affinity towards human 5-hydroxytryptamine 7 receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
Virginia Commonwealth University
Curated by ChEMBL
Virginia Commonwealth University
Curated by ChEMBL
Target5-hydroxytryptamine receptor 7(Rattus norvegicus (rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
TargetSigma non-opioid intracellular receptor 1(Cavia porcellus (Guinea pig))
TBA
Curated by PDSP Ki Database
TBA
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2C(Rattus norvegicus (Rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
TargetAlpha-2C adrenergic receptor(Homo sapiens (Human))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
TargetAlpha-2B adrenergic receptor(Homo sapiens (Human))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
TargetAlpha-2A adrenergic receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetAlpha-2A adrenergic receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetAlpha-2A adrenergic receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1A/1B/1D/2C(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetNADPH oxidase 1(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 1.70E+4nMAssay Description:Data Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institut...More data for this Ligand-Target Pair
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.34E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
TargetBcl-2-related protein A1(Mus musculus (Mouse))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 2.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.62E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
Affinity DataIC50: 1.06E+4nMAssay Description:Inhibition of human recombinant CYP2J2 assessed as reduction in astemizole O-demethylation by LC-MS/MS methodMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using bufuralol substrate by LC-MS/MS methodMore data for this Ligand-Target Pair