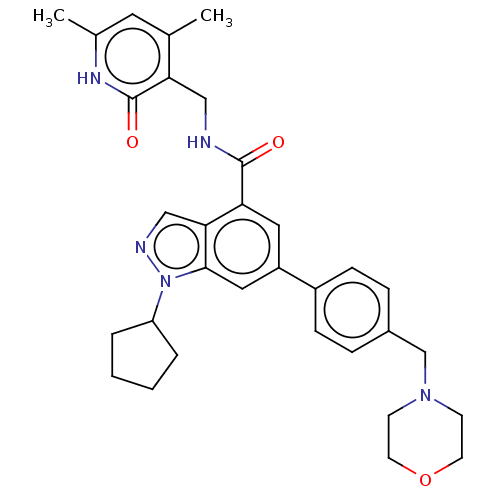
null
SMILES Cc1cc(C)c(CNC(=O)c2cc(cc3n(ncc23)C2CCCC2)-c2ccc(CN3CCOCC3)cc2)c(=O)[nH]1
InChI Key InChIKey=ZOIBZSZLMJDVDQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 50075051
Found 17 hits for monomerid = 50075051
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:Competitive inhibition of human EZH2 using SAM and histone H3 (16 to 30) as substrate preincubated for 30 mins followed by substrate addition measure...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:Competitive inhibition of wild type EZH2 in human PRC2 complex using S-adenosylmethionine as substrate by Michaelis-Menten plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 11.3nMAssay Description:Test compounds were serially diluted 3-fold in DMSO in a 10 point-curve and 1 uL was spotted into a 384-well microplate in duplicate using a Platemat...More data for this Ligand-Target Pair
Affinity DataIC50: 8.20nMAssay Description:Test compounds were serially diluted 3-fold in DMSO in a 10 point-curve and 1 uL was spotted into a 384-well microplate in duplicate using a Platemat...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 5.40E+4nMAssay Description:Inhibition of human EZH2 using SAM and histone H3 (16 to 30) as substrate preincubated for 30 mins followed by substrate addition measured after 90 m...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of wild-type EZH2 in human OCI-LY19 cells assessed as reduction of H3K27me3 level after 96 hrs by ELISA assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Affinity DataEC50: 2.90E+3nMAssay Description:Inhibition of methyltransferase activity of EZH2 in human G401 cells assessed as H3K27 trimethylation after 4 hrs by ELISAMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Recombinant PRC2 Enzymes. Human PRC2 enzymes were purified as 4-component enzyme complexes co-expressed in Spodoptera frugiperda (sf9) cells using a ...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:eneral Procedure for Wild-Type PRC2 Enzyme Assay on Oligonucleosome Substrate. The assays was performed in a buffer consisting of 20 mM bicine (pH=7....More data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of EZH1 in PRC2 complex (unknown origin) using biotinylated peptide as substrate in presence of S-adenosylmethionineMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 54nMAssay Description:Inhibition of EZH2 in human PRC2 complex preincubated for 30 mins followed by histone h3 peptide (21 to 44 residues) and S-adenosylmethionine and mea...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
Affinity DataIC50: 95nMAssay Description:Test compounds were serially diluted 3-fold in DMSO in a 10 point-curve and 1 uL was spotted into a 384-well microplate in duplicate using a Platemat...More data for this Ligand-Target Pair
Affinity DataIC50: 75nMAssay Description:Test compounds were serially diluted 3-fold in DMSO in a 10 point-curve and 1 uL was spotted into a 384-well microplate in duplicate using a Platemat...More data for this Ligand-Target Pair