null
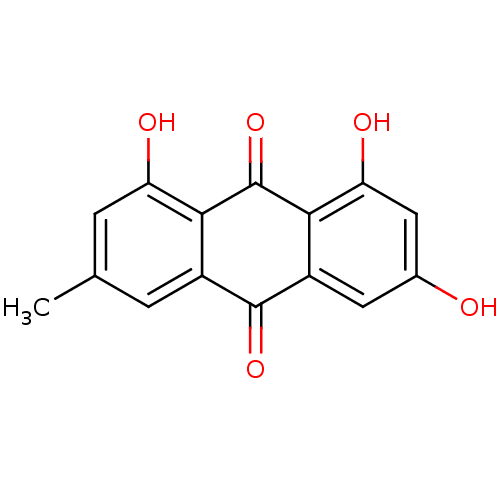
SMILES Cc1cc(O)c2C(=O)c3c(O)cc(O)cc3C(=O)c2c1
InChI Key InChIKey=RHMXXJGYXNZAPX-UHFFFAOYSA-N
PDB links: 14 PDB IDs match this monomer. 2 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 45 hits for monomerid = 11318
Found 45 hits for monomerid = 11318
Affinity DataKi: 770nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor betaMore data for this Ligand-Target Pair
TargetCasein kinase II subunit alpha(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 1.50E+3nMAssay Description:Inhibition of CK2alpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.85E+3nM ΔG°: -7.74kcal/molepH: 7.5 T: 2°CAssay Description:In vitro kinase assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP/ [gam...More data for this Ligand-Target Pair
TargetCasein kinase II subunit alpha(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 1.85E+3nM ΔG°: -7.74kcal/mole IC50: 890nMpH: 7.5 T: 2°CAssay Description:In vitro kinase assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP/ [gam...More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+3nMAssay Description:Inhibition of CK2 (unknown origin)More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
University of Colorado
Curated by ChEMBL
University of Colorado
Curated by ChEMBL
Affinity DataKi: 2.11E+3nMAssay Description:Uncompetitive inhibition of human recombinant aldose reductase expressed in Escherichia coli using DL-glyceraldehyde as substrate by double reciproca...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]PSB0413 from human platelet P2Y12 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+5nMAssay Description:Inhibition of human Neu2 assessed as MuNANA substrate hydrolysis in presence of 0.1% Triton X-100 by discontinuous fluorimetric assayMore data for this Ligand-Target Pair
TargetProtein tyrosine phosphatase type IVA 3(Homo sapiens (Human))
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of PRL3 after 1 hr by DiFMUP assayMore data for this Ligand-Target Pair
TargetProtein tyrosine phosphatase type IVA 3(Homo sapiens (Human))
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of PRL-3-mediated cell migration in human DLD1 cells after 15 hrs by crystal violet staining based microscopic assayMore data for this Ligand-Target Pair
TargetProtein tyrosine phosphatase type IVA 3(Homo sapiens (Human))
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Affinity DataIC50: 3.50E+4nMAssay Description:Inhibition of PRL-3-mediated cell invasion in human DLD1 cells after 20 hrs using crystal violet staining by Matrigel invasion assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alphaMore data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
School of Chemistry and Biochemistry
Curated by ChEMBL
School of Chemistry and Biochemistry
Curated by ChEMBL
Affinity DataIC50: 5.20E+4nMAssay Description:Compound was tested for the enzyme inhibitory activity against Human Leukocyte Elastase (HLE) at 53 uM concentrationMore data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+3nMAssay Description:Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alphaMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
University of Colorado
Curated by ChEMBL
University of Colorado
Curated by ChEMBL
Affinity DataIC50: 2.69E+3nMAssay Description:Inhibition of human recombinant aldose reductase expressed in Escherichia coli using DL-glyceraldehyde as substrate in presence of NADPH by spectroph...More data for this Ligand-Target Pair
In DepthDetails
TargetCytochrome P450 1A1(Homo sapiens (Human))
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Affinity DataIC50: 1.23E+4nMAssay Description:Inhibition of recombinant human CYP1A1 expressed in supersomes using 7-ethoxyresorufin O-deethylation as substrate after 5 mins in presence of NADP+ ...More data for this Ligand-Target Pair
TargetCasein kinase II subunit alpha/beta(Homo sapiens (Human))
Instituto Universitario de Bio-Org£nica Antonio Gonz£lez (CIBICAN)
Curated by ChEMBL
Instituto Universitario de Bio-Org£nica Antonio Gonz£lez (CIBICAN)
Curated by ChEMBL
Affinity DataIC50: 580nMAssay Description:Inhibition of human CK2-alpha/beta expressed in Escherichia coli BL21(DE3) using RRRDDDSDDD peptide as substrate after 15 mins by capillary electroph...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataEC50: 2.00E+5nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataIC50: 1.69E+4nMAssay Description:Inhibition of Dengue virus type 2 NS5 RNA methyltransferase SAM siteMore data for this Ligand-Target Pair
Affinity DataIC50: 7.98E+4nMAssay Description:Inhibition of Dengue virus type 2 NS5 RNA methyltransferase SAM site with 0.1 % TX100More data for this Ligand-Target Pair
TargetProteasome subunit beta type-2(Homo sapiens (Human))
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
Affinity DataIC50: 2.09E+4nMAssay Description:Inhibition of trypsin-like activity of human 26S proteasome assessed as decrease in AMC hydrolysis using Ac-Arg-Leu-Arg-AMC as substrate incubated fo...More data for this Ligand-Target Pair
TargetProteasome subunit beta type-5(Homo sapiens (Human))
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
Affinity DataIC50: 1.22E+3nMAssay Description:Inhibition of chymotrypsin-like activity of human 26S proteasome assessed as decrease in AMC hydrolysis using Suc-Leu-Leu-Val-Tyr-AMC as substrate in...More data for this Ligand-Target Pair
TargetProteasome subunit beta type-1(Homo sapiens (Human))
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
Affinity DataIC50: 240nMAssay Description:Inhibition of caspase-like activity of human 26S proteasome assessed as decrease in fluorescence using Z-Nle-Pro-Nle-Asp-aminoluciferin as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of bovine kidney alpha-fucosidase using PNPG as substrate incubated for 10 mins by spectrophotometric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ...More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
TargetCytochrome P450 1A1(Homo sapiens (Human))
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
In DepthDetails
In DepthDetails
In DepthDetails
TargetSerine/threonine-protein kinase pim-1(Homo sapiens (Human))
Xavier University of Louisiana
Curated by ChEMBL
Xavier University of Louisiana
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of recombinant human Pim1More data for this Ligand-Target Pair
TargetCasein kinase II subunit alpha(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of GST-fused human recombinant CK2alpha expressed in Escherichia coli HMS174 (DE3)More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 3.64E+4nMAssay Description:Inhibition of sEH (unknown origin) assessed as 6-methoxy-2-naphthaldehyde formation by fluorometry assay using 40 uM cyano-(6-methoxy-naphthalen-2-yl...More data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+4nMAssay Description:Inhibition of p56 lckMore data for this Ligand-Target Pair
TargetAccessory gene regulator protein A(Staphylococcus aureus)
University of North Carolina at Greensboro
Curated by ChEMBL
University of North Carolina at Greensboro
Curated by ChEMBL
Affinity DataIC50: 1.71E+4nMAssay Description:Inhibition of AGR quorum sensing system in methicillin-resistant Staphylococcus aureus AH2759 incubated for 15 hrs by P3-LUX reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+5nMAssay Description:Inhibitory activity against HRV 3Cpro using HPLC assayMore data for this Ligand-Target Pair
Target3-hydroxyacyl-[acyl-carrier-protein] dehydratase FabZ(Francisella tularensis)
Brookhaven National Laboratory
Brookhaven National Laboratory
Affinity DataIC50: 4.31E+4nMpH: 7.0 T: 2°CAssay Description:The enzymatic activities of FtFabZ and YpFabZ were determined via the reportedspectrophotometric method using the substrate analogue crotonoyl-CoA.7 ...More data for this Ligand-Target Pair
Target3-hydroxyacyl-[acyl-carrier-protein] dehydratase FabZ [1-175](Yersinia pestis)
Brookhaven National Laboratory
Brookhaven National Laboratory
Affinity DataIC50: 2.97E+4nMpH: 7.0 T: 2°CAssay Description:The enzymatic activities of FtFabZ and YpFabZ were determined via the reportedspectrophotometric method using the substrate analogue crotonoyl-CoA.7 ...More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Affinity DataIC50: 3.73E+3nMAssay Description:Inhibition of recombinant human CYP1A2 expressed in supersomes using 7-ethoxyresorufin O-deethylation as substrate after 5 mins in presence of NADP+ ...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)