null
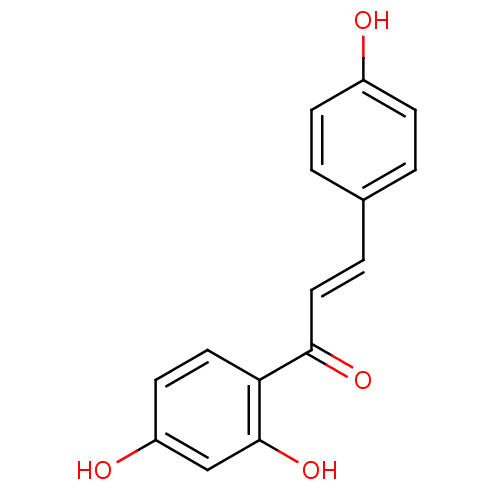
SMILES Oc1ccc(\C=C\C(=O)c2ccc(O)cc2O)cc1
InChI Key InChIKey=DXDRHHKMWQZJHT-FPYGCLRLSA-N
PDB links: 5 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 42 hits for monomerid = 50042944
Found 42 hits for monomerid = 50042944
TargetBeta-secretase 1(Homo sapiens (Human))
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
Affinity DataIC50: 3.30E+4nMpH: 4.5 T: 2°CAssay Description:The assay based on fluorescenceresonance energy transfer was carried out with BACE1 enzyme at pH 4.5 with a substrate, H-Lys(DABSYL)-SEVNLDAEFR-Gin-(...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Rattus norvegicus)
Tokushima Bunri University
Curated by ChEMBL
Tokushima Bunri University
Curated by ChEMBL
Affinity DataIC50: 3.50E+4nMAssay Description:In vitro inhibition against 5-lipoxygenase in RBL-1 cells was determinedMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Homo sapiens (Human))
Instituto de Qu£mica M£dica (IQM-CSIC)
Curated by ChEMBL
Instituto de Qu£mica M£dica (IQM-CSIC)
Curated by ChEMBL
Affinity DataEC50: 1.16E+4nMAssay Description:Positive allosteric modulation at human alpha7 nACHR expressed in Xenopus oocyte assessed as potentiation of 200 uM ACh-induced current at holding po...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
King's College London
Curated by ChEMBL
King's College London
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:Inhibition of human recombinant aldose reductase using D-glyceraldehyde as substrate preincubated for 10 mins before substrate addition measured for ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Compound concentration required to reduce HIV-1 Integrase 3'-processing activity by 50%More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Rattus norvegicus)
Tokushima Bunri University
Curated by ChEMBL
Tokushima Bunri University
Curated by ChEMBL
Affinity DataIC50: 3.50E+4nMAssay Description:Inhibition of 5-lipoxygenase in rat RBL1 cellsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Institute
Curated by ChEMBL
Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of recombinant human PTP1B assessed as hydrolysis of p-nitrophenyl phosphate after 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Institute
Curated by ChEMBL
Institute
Curated by ChEMBL
Affinity DataIC50: 6.00E+4nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against HIV-1 Integrase (HIV-1-IN)More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
North-West University
Curated by ChEMBL
North-West University
Curated by ChEMBL
Affinity DataIC50: 1.73E+4nMAssay Description:Inhibition of Wistar rat liver mitochondrial MAO-A using benzylamine hydrochloride as substrate after 1 hr by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1/2(Homo sapiens (Human))
Tokushima Bunri University
Curated by ChEMBL
Tokushima Bunri University
Curated by ChEMBL
Affinity DataIC50: 1.30E+5nMAssay Description:Inhibition of Prostaglandin G/H synthase activity in sheep seminal vesicle was determined 100 uMMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Bos taurus (Bovine))
Guangxi Medical University
Curated by ChEMBL
Guangxi Medical University
Curated by ChEMBL
Affinity DataIC50: 4.73E+4nMAssay Description:Inhibition of bovine milk xanthine oxidase pre-incubated for 30 mins followed by xanthine addition and measured every 30 secs for 5 mins by spectroph...More data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
Affinity DataIC50: 5.15E+4nMAssay Description:Ability to displace [3H]quipazine binding to 5-hydroxytryptamine 3 receptor sites in NG 108-15.More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 2(Homo sapiens (Human))
University of Basel
Curated by ChEMBL
University of Basel
Curated by ChEMBL
Affinity DataIC50: 360nMAssay Description:Inhibition of human 17beta-HSD2 expressed in HEK293 cell lysates incubated for 10 mins using [2,4,6,7-3H]-estradiol and NAD+ by scintillation countin...More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Basel
Curated by ChEMBL
University of Basel
Curated by ChEMBL
Affinity DataIC50: 2.83E+3nMAssay Description:Inhibition of human 17beta-HSD1 expressed in HEK293 cell lysates incubated for 10 mins using [2,4,6,7-3H]-estrone and NADPH by scintillation counting...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of aromatase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.87E+3nMAssay Description:Displacement of estradiol from human ERalpha expressed in yeast cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 269nMAssay Description:Displacement of estradiol from human ERbetaa expressed in yeast cellsMore data for this Ligand-Target Pair
TargetNACHT, LRR and PYD domains-containing protein 3(Homo sapiens (Human))
Soochow University
Curated by ChEMBL
Soochow University
Curated by ChEMBL
Affinity DataIC50: 1.54E+3nMAssay Description:Inhibition of NLRP3 inflammasome in human THP1 cells assessed as reduction in MSU-induced IL-1beta production preincubated for 1 hr followed by MSU s...More data for this Ligand-Target Pair
Affinity DataIC50: 1.32E+4nMAssay Description:Inhibition of oseltamivir-resistant H1N1 swine influenza virus neuraminidase H274Y mutant activity expressed in HEK293T cells after 2 hrs by spectrof...More data for this Ligand-Target Pair
Affinity DataIC50: 3.78E+4nMAssay Description:Inhibition of Influenza A H9N2 virus neuraminidase activity after 2 hrs by spectrofluorometryMore data for this Ligand-Target Pair
Affinity DataIC50: 4.29E+4nMAssay Description:Noncompetitive inhibition of Influenza A H1N1 virus neuraminidase activity by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.27E+4nMAssay Description:Inhibition of Influenza A H1N1 virus neuraminidase activity after 2 hrs by spectrofluorometryMore data for this Ligand-Target Pair
Affinity DataIC50: 4.29E+4nMAssay Description:Inhibition of Clostridium perfringens neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.36E+4nMAssay Description:Inhibition of wild type H1N1 swine influenza virus neuraminidase activity expressed in HEK293T cells after 2 hrs by spectrofluorometryMore data for this Ligand-Target Pair
TargetBeta-secretase 1(Homo sapiens (Human))
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of Rattus norvegicus (rat) lens aldose reductaseMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
North-West University
Curated by ChEMBL
North-West University
Curated by ChEMBL
Affinity DataIC50: 4.72E+4nMAssay Description:Displacement of [14C]-beta-PEA from rat MAO-B after 20 mins by liquid scintillation counting analysisMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
North-West University
Curated by ChEMBL
North-West University
Curated by ChEMBL
Affinity DataIC50: 1.39E+4nMAssay Description:Displacement of [14C]-5HT from rat MAO-A after 20 mins by liquid scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.97E+4nMAssay Description:Inhibition of amyloid beta (1-42) self-mediated aggregation (unknown origin) after 5 days by thioflavin T fluorescence methodMore data for this Ligand-Target Pair
TargetProteasome subunit beta type-5(Homo sapiens (Human))
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
Affinity DataIC50: 4.88E+3nMAssay Description:Inhibition of chymotrypsin-like activity of purified human erythrocyte 20S proteasome assessed as decrease in AMC hydrolysis using Suc-LLVY-AMC as su...More data for this Ligand-Target Pair
TargetNACHT, LRR and PYD domains-containing protein 3(Homo sapiens (Human))
Soochow University
Curated by ChEMBL
Soochow University
Curated by ChEMBL
Affinity DataIC50: 1.01E+4nMAssay Description:Inhibition of NLRP3 in human THP1 cells assessed as inhibition of MSU-induced IL-1beta productionMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Inhibition of TDP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) using 5'-FAM-AGGATCTAAAAGACTT-BHQ-3' as substrate preincubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataEC50: >3.50E+5nMAssay Description:Keywords: apoptosis, BH3 domain, Bcl2-A1, BIM, caspase, cancer Primary Collaborator: Todd Golub, Broad Institute, golub@broadinstitute.org Assay Over...More data for this Ligand-Target Pair
TargetProbable global transcription activator SNF2L2(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay