null
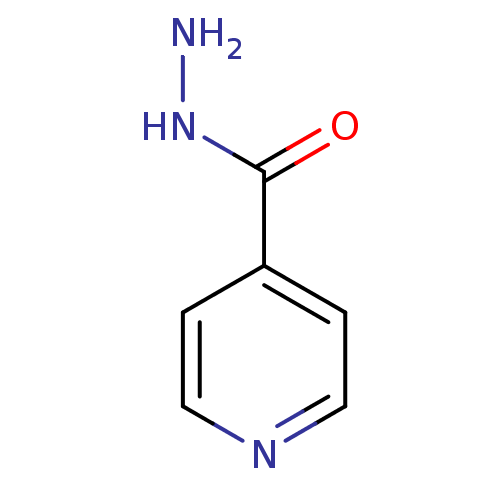
SMILES NNC(=O)c1ccncc1
InChI Key InChIKey=QRXWMOHMRWLFEY-UHFFFAOYSA-N
PDB links: 12 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 30 hits for monomerid = 50336507
Found 30 hits for monomerid = 50336507
Affinity DataKi: 1nMAssay Description:Inhibition of Mycobacterium tuberculosis dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Mechanism based inhibition of human cytochrome P450 2C19 evaluated using TolbutamideMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+4nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone 6-beta hydroxylation using human liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 5.60E+4nMAssay Description:Mechanism based inhibition of human cytochrome P450 1A2 measured by formation of 6-hydroxywarfarinMore data for this Ligand-Target Pair
Affinity DataKi: 6.00E+4nMAssay Description:Mechanism based inhibition of human cytochrome P450 2A6 measured by coumarin 7-hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+5nMAssay Description:Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using human liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 3.74E+5nMAssay Description:Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using a recombinant systemMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Irreversible inhibition of MPO (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibition of human MPOMore data for this Ligand-Target Pair
Affinity DataIC50: 1.35E+5nMAssay Description:Inhibitory activity against thymidine monophosphate kinase (TMPK) in Mycobacterium tuberculosisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
TargetPantothenate synthetase(Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
Birla Institute of Technology & Science-Pilani
Curated by ChEMBL
Birla Institute of Technology & Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis pantothenate synthetase expressed in Escherichia coli BL21 (DE3) by spectrophotometryMore data for this Ligand-Target Pair
TargetDNA gyrase subunit B(Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
Institute of Technology & Science-Pilani
Curated by ChEMBL
Institute of Technology & Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis DNA gyrase assessed as inhibition of DNA supercoiling after 30 mins by electrophoresisMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Kyungpook National University
Curated by ChEMBL
Kyungpook National University
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of mushroom tyrosinase using L-tyrosine as substrateMore data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH](Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
Nanjing University
Curated by ChEMBL
Nanjing University
Curated by ChEMBL
Affinity DataIC50: 8.29E+3nMAssay Description:Inhibition of recombinant Mycobacterium tuberculosis InhA expressed in Escherichia coli Rosette(DE3) pLysS using trans-2-decenoyl-N-acetylcysteamine ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of Escherichia coli DNA gyrase assessed as reduction in enzyme-mediated supercoiling of relaxed pBR322 DNA measured after 60 mins by elect...More data for this Ligand-Target Pair
Target3-oxoacyl-[acyl-carrier-protein] synthase 3(Escherichia coli)
Nanjing University
Curated by ChEMBL
Nanjing University
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of Escherichia coli KAS3 expressed in Escherichia coli BL21(DE3)More data for this Ligand-Target Pair
TargetPantothenate synthetase(Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
Birla Institute of Technology & Science-Pilani
Curated by ChEMBL
Birla Institute of Technology & Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of Sprague-Dawley rat Bsep expressed in plasma membrane vesicles of Sf21 cells assessed as inhibition of ATP-dependent [3H]taurocholate up...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of human BSEP expressed in plasma membrane vesicles of Sf21 cells assessed as inhibition of ATP-dependent [3H]taurocholate uptakeMore data for this Ligand-Target Pair
TargetIsocitrate lyase(Mycobacterium tuberculosis)
Birla Institute of Technology & Science-Pilani, Hyderabad Campus
Birla Institute of Technology & Science-Pilani, Hyderabad Campus
Affinity DataIC50: 4.56E+5nMT: 2°CAssay Description:Isocitrate lyase activity was determined at 37°C by measuringthe formation of glyoxylate phenylhydrazone at 324 nm [Bai et al., Drug Dev. Res., 6...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of MymA activity by INH was checked using different concentrations (0┐12 μm) of INH, 100 μm of trimeth- ylamine and 5 mg of MymA...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMpH: 7.5 T: 2°CAssay Description:A total of 4 ÁL of OGG1 (125 nM) was mixed with 1 ÁL of inhibitor. In a separate tube, 1 ÁL of freshly prepared 10 mM NaBH3CN (diluted in H2O) was mi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Gel-based assays were performed by combining 4 ÁL of OGG1 (62.5 nM) with 1 ÁL of inhibitor or buffer. Substrate (5 ÁL, 50 nM) was added to bring the ...More data for this Ligand-Target Pair
TargetPantothenate synthetase(Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
Birla Institute of Technology & Science-Pilani
Curated by ChEMBL
Birla Institute of Technology & Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis pantothenate synthetase expressed in Escherichia coli BL21 (DE3)More data for this Ligand-Target Pair