null
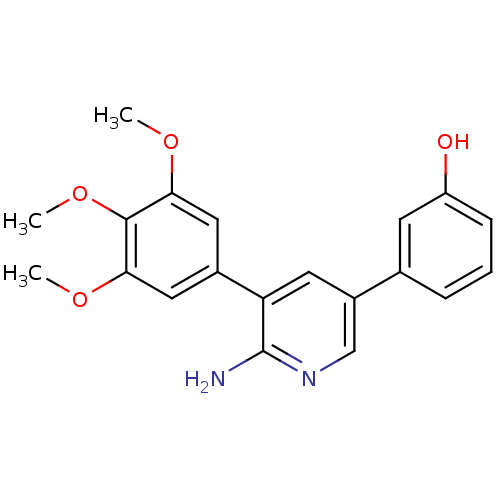
SMILES COc1cc(cc(OC)c1OC)-c1cc(cnc1N)-c1cccc(O)c1
InChI Key InChIKey=CJLMANFTWLNAKC-UHFFFAOYSA-N
PDB links: 2 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 102619
Found 16 hits for monomerid = 102619
TargetSerine/threonine-protein kinase receptor R3(Mus musculus)
Massachusetts Institute of Technology
Massachusetts Institute of Technology
TargetBone morphogenetic protein receptor type-1A(Mus musculus)
Massachusetts Institute of Technology
Massachusetts Institute of Technology
TargetActivin receptor type-1(Homo sapiens (Human))
Massachusetts Institute of Technology
Curated by ChEMBL
Massachusetts Institute of Technology
Curated by ChEMBL
Affinity DataIC50: 35nMAssay Description:Inhibition of human recombinant human ALK2 kinase after 45 mins by liquid scintillation counting in presence of ATP [gamma-32P]More data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Homo sapiens (Human))
Massachusetts Institute of Technology
Curated by ChEMBL
Massachusetts Institute of Technology
Curated by ChEMBL
Affinity DataIC50: 280nMAssay Description:Inhibition of purified human ALK5 kinase after 45 mins by liquid scintillation counting in presence of ATP [gamma-32P]More data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Homo sapiens (Human))
Massachusetts Institute of Technology
Curated by ChEMBL
Massachusetts Institute of Technology
Curated by ChEMBL
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of TGFbeta1-induced TGFbeta type 1 ALK5 in HEK293T cells after 30 mins by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 230nMAssay Description:Inhibition of BMP2-induced BMP receptor type 1 ALK2 in mouse C2C12 cells after 30 mins by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 340nMAssay Description:Inhibition of BMP4-induced BMP receptor in mouse C2C12 cells after 30 mins by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 420nMAssay Description:Inhibition of BMP6-induced BMP receptor type 1 ALK2 in mouse C2C12 cells after 30 mins by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 723nMAssay Description:The assay procedure determines the IC50 of each potential FYN kinase inhibitor by measuring the enzyme catalyzed ATP-dependent phosphorylation of the...More data for this Ligand-Target Pair
Affinity DataIC50: 526nMAssay Description:The assay procedure determines the IC50 of each potential FYN kinase inhibitor by measuring the enzyme catalyzed ATP-dependent phosphorylation of the...More data for this Ligand-Target Pair
TargetActivin receptor type-1(Homo sapiens (Human))
Massachusetts Institute of Technology
Curated by ChEMBL
Massachusetts Institute of Technology
Curated by ChEMBL
 3D Structure (crystal)
3D Structure (crystal)