null
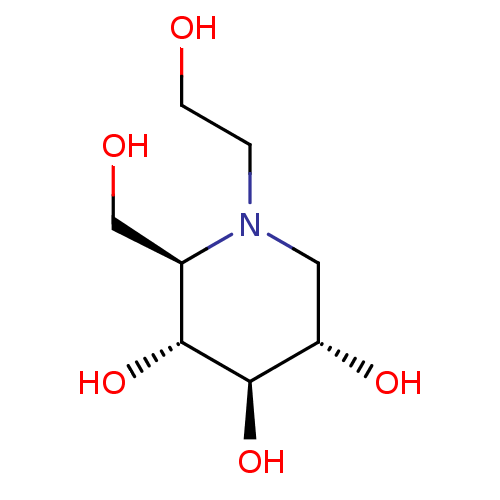
SMILES OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO
InChI Key InChIKey=IBAQFPQHRJAVAV-ULAWRXDQSA-N
PDB links: 4 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 41 hits for monomerid = 50242271
Found 41 hits for monomerid = 50242271
Affinity DataKi: 460nMAssay Description:Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
TargetBeta-galactosidase(Bos taurus (Bovine))
Savitribai Phule Pune University (formerly University of Pune)
Curated by ChEMBL
Savitribai Phule Pune University (formerly University of Pune)
Curated by ChEMBL
Affinity DataKi: 1.00E+5nMAssay Description:Inhibition of bovine liver beta-galactosidase using 10 mM p-nitrophenyl-beta-D-galactopyranoside as substrateMore data for this Ligand-Target Pair
TargetSucrase-isomaltase, intestinal(Rattus norvegicus (Rat))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 3.90E+4nMAssay Description:Inhibition of Wistar rat intestinal isomaltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetSucrase-isomaltase, intestinal(Rattus norvegicus (Rat))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetBeta-galactosidase(Bos taurus (Bovine))
Savitribai Phule Pune University (formerly University of Pune)
Curated by ChEMBL
Savitribai Phule Pune University (formerly University of Pune)
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of bovine liver beta-galactosidase using 10 mM p-nitrophenyl-beta-D-galactopyranoside as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of human small intestine microsomal maltase using maltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
TargetSucrase-isomaltase, intestinal(Rattus norvegicus (Rat))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 430nMAssay Description:Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
TargetSucrase-isomaltase, intestinal(Rattus norvegicus (Rat))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 4.60E+3nMAssay Description:Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
TargetOligo-1,6-glucosidase IMA1(Saccharomyces cerevisiae S288c (Baker's yeast))
Martin-Luther-Universit£t Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-Universit£t Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 9.90E+6nMAssay Description:Inhibition of baker's yeast alpha-glucosidase by 4-nitrophenolate-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of GCS by cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of lysosomal alpha-glucosidase by HPLCMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of maltase by HPLCMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of lactase by HPLCMore data for this Ligand-Target Pair
Affinity DataIC50: 590nMAssay Description:Inhibition of Wistar rat small intestine maltase after 30 minsMore data for this Ligand-Target Pair
TargetSucrase-isomaltase, intestinal(Rattus norvegicus (Rat))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 3.90E+4nMAssay Description:Inhibition of Wistar rat small intestine isomaltase after 30 minsMore data for this Ligand-Target Pair
TargetSucrase-isomaltase, intestinal(Rattus norvegicus (Rat))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of Wistar rat small intestine sucrase after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of rat intestinal brush border membrane maltaseMore data for this Ligand-Target Pair
TargetSucrase-isomaltase, intestinal(Rattus norvegicus (Rat))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of rat intestinal brush border membrane isomaltaseMore data for this Ligand-Target Pair
TargetSucrase-isomaltase, intestinal(Rattus norvegicus (Rat))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of rat intestinal brush border membrane sucraseMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibition of human lysosomal alpha-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 8.40E+4nMAssay Description:Inhibition of human lysosomal beta-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 390nMAssay Description:Inhibition of rabbit muscle amylo-1,6-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of maltase in human Caco-2 cell model system after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of rat intestinal maltase using moltose as substrateMore data for this Ligand-Target Pair
TargetSucrase-isomaltase, intestinal(Rattus norvegicus (Rat))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of rat intestinal sucrase using sucrose as substrateMore data for this Ligand-Target Pair
TargetSucrase-isomaltase, intestinal(Rattus norvegicus (Rat))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 3.90E+4nMAssay Description:Inhibition of rat intestinal isomaltase using isomoltose as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
TargetAlpha-glucosidase(Aspergillus niger)
Savitribai Phule Pune University (Formerly
Curated by ChEMBL
Savitribai Phule Pune University (Formerly
Curated by ChEMBL
Affinity DataIC50: 5.71E+4nMAssay Description:Inhibition of Aspergillus niger alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 1 hr followed by substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 7.59E+4nMAssay Description:Inhibition of Canavalia ensiformis alpha-mannosidase using p-nitrophenyl-alpha-D-mannopyranoside as substrate preincubated for 1 hr followed by subst...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of human intestinal maltase using maltose as substrate incubated for 30 mins and immediately heated for 2 mins by glucose oxidase methodMore data for this Ligand-Target Pair
Affinity DataKd: 1.05E+5nMAssay Description:Inhibition of jack bean alpha-mannosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of Wistar rat intestinal maltase assessed as inhibition of D-glucose release after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 3.79E+5nMAssay Description:Inhibition of alpha-mannosidase (unknown origin) using p-nitrophenyl-alpha-D-mannopyranoside substrate incubated for 10 mins by UV spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.54E+5nMAssay Description:In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont...More data for this Ligand-Target Pair
TargetApelin receptor(Homo sapiens (Human))
Sanford-Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Sanford-Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 3.17E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford- Sanford-Burnham Medical Research Institute(SBMRI, San...More data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of sucrase by HPLCMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)