null
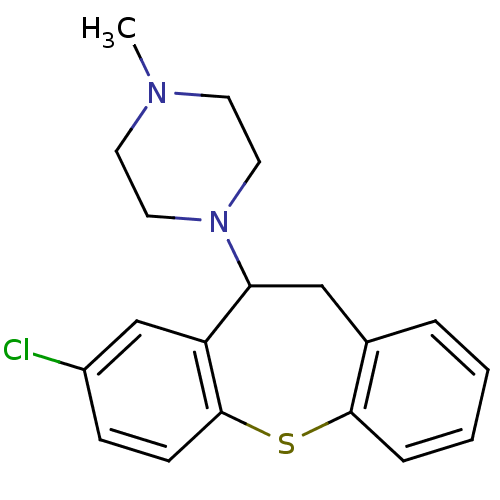
SMILES CN1CCN(CC1)C1Cc2ccccc2Sc2ccc(Cl)cc12
InChI Key InChIKey=XRYLGRGAWQSVQW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 35 hits for monomerid = 22872
Found 35 hits for monomerid = 22872
Affinity DataKi: 0.190nMAssay Description:Displacement of [3H]prozosin from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Universit£ degli Studi di Siena
Curated by ChEMBL
Universit£ degli Studi di Siena
Curated by ChEMBL
Affinity DataKi: 0.230nMAssay Description:Half-maximal inhibition of [3H]ketanserin binding to 5-hydroxytryptamine 2A receptor in rat cerebral cortex homogenateMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Rattus norvegicus (rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
TargetD(2) dopamine receptor(Rattus norvegicus (rat))
Universit£ degli Studi di Siena
Curated by ChEMBL
Universit£ degli Studi di Siena
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Half-maximal inhibition of [3H]spiperone binding to Dopamine receptor D2 in rat striatal homogenateMore data for this Ligand-Target Pair
Affinity DataKi: 0.560nMAssay Description:Displacement of [3H]prozosin from hamster cloned alpha1b receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
University of Copenhagen
Curated by ChEMBL
University of Copenhagen
Curated by ChEMBL
Affinity DataKi: 0.570nMAssay Description:Displacement of [3H]prozosin from human cloned 5HT2C receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.660nMAssay Description:Displacement of [3H]prozosin from bovine cloned alpha1a receptor expressed in BHK cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.670nMAssay Description:Displacement of [3H]prozosin from human cloned dopamine D2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAlpha-1D adrenergic receptor(Rattus norvegicus (Rat))
University of Copenhagen
Curated by ChEMBL
University of Copenhagen
Curated by ChEMBL
Affinity DataKi: 0.770nMAssay Description:Displacement of [3H]prozosin from rat cloned alpha1d receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Rattus norvegicus (rat))
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
TargetD(2) dopamine receptor(Rattus norvegicus (rat))
Universit£ degli Studi di Siena
Curated by ChEMBL
Universit£ degli Studi di Siena
Curated by ChEMBL
Target5-hydroxytryptamine receptor 6(Homo sapiens (Human))
University of Washington
Curated by PDSP Ki Database
University of Washington
Curated by PDSP Ki Database
Affinity DataKi: 2.30nMAssay Description:Half-maximal inhibition of [3H]-SCH- 23390 binding to Dopamine receptor D1 in rat striatal homogenateMore data for this Ligand-Target Pair
TargetD(3) dopamine receptor(Rattus norvegicus (Rat))
Universit£ degli Studi di Siena
Curated by ChEMBL
Universit£ degli Studi di Siena
Curated by ChEMBL
Affinity DataKi: 2.40nMAssay Description:Half-maximal inhibition of [3H]-7-OH-DPAT binding to Dopamine receptor D3 in rat tissue homogenateMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(GUINEA PIG)
Syntex Discovery Research
Curated by PDSP Ki Database
Syntex Discovery Research
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 6(Homo sapiens (Human))
University of Washington
Curated by PDSP Ki Database
University of Washington
Curated by PDSP Ki Database
TargetD(2) dopamine receptor(Rattus norvegicus (rat))
Universit£ degli Studi di Siena
Curated by ChEMBL
Universit£ degli Studi di Siena
Curated by ChEMBL
Target5-hydroxytryptamine receptor 7(GUINEA PIG)
Syntex Discovery Research
Curated by PDSP Ki Database
Syntex Discovery Research
Curated by PDSP Ki Database
TargetTrace amine-associated receptor 1(Homo sapiens (Human))
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
Affinity DataKi: >1.00E+4nM ΔG°: >-7.09kcal/molepH: 7.4 T: 2°CAssay Description:Ligand displacement assays were performed on The SK-N-MC/hH4R cell homogenates. Retained radioactivity was determined by liquid scintillation countin...More data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 9(Homo sapiens (Human))
Universidad de Talca
Curated by ChEMBL
Universidad de Talca
Curated by ChEMBL
Affinity DataIC50: 7.38E+4nMAssay Description:Inhibition of human TASK3 expressed in HEK293 cells by Ti+ flux assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Ability to displace [3H]haloperidol from rat striatal membranes, in order to measure its intrinsic affinity for the dopamine (DA) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Ability to inhibit binding of [3H]-SCH- 23390 to Dopamine receptor D1 in rat striatal membranesMore data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Rattus norvegicus (rat))
Universit£ degli Studi di Siena
Curated by ChEMBL
Universit£ degli Studi di Siena
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Ability to inhibit binding of [3H]-SPI to dopamine receptor D2 in rat striatal membranesMore data for this Ligand-Target Pair
TargetAlpha-1A/Alpha-1B/Alpha-1D adrenergic receptor(Rattus norvegicus (rat))
H. Lundbeck A/S
Curated by ChEMBL
H. Lundbeck A/S
Curated by ChEMBL
Affinity DataIC50: 0.180nMAssay Description:Ability to inhibit binding of [3H]PRAZ to alpha-1 adrenergic receptor in rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 0.570nMAssay Description:Ability to inhibit binding of [3H]KET to 5-hydroxytryptamine 2 receptor in rat cortexMore data for this Ligand-Target Pair
TargetPotassium channel subfamily K member 9(Homo sapiens (Human))
Universidad de Talca
Curated by ChEMBL
Universidad de Talca
Curated by ChEMBL
Affinity DataIC50: 7.38E+4nMAssay Description:Inhibition of human TASK3 expressed in HEK293 cells by Ti+ flux assayMore data for this Ligand-Target Pair
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 2.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair