null
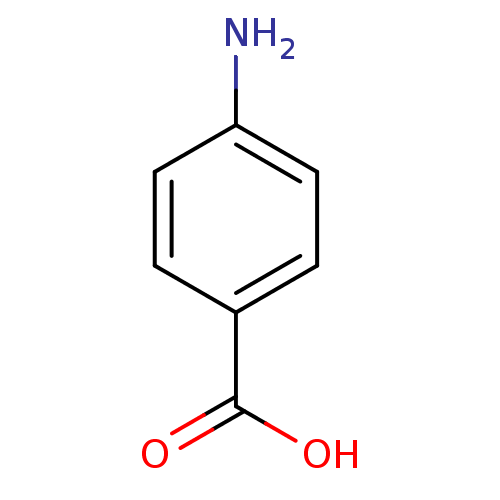
SMILES Nc1ccc(cc1)C(O)=O
InChI Key InChIKey=ALYNCZNDIQEVRV-UHFFFAOYSA-N
PDB links: 13 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50145829
Found 8 hits for monomerid = 50145829
Affinity DataKd: >4.00E+4nMAssay Description:Dissociation constant for HCV NS3 protease substrate binding siteMore data for this Ligand-Target Pair
TargetSuccinate-semialdehyde dehydrogenase, mitochondrial(Homo sapiens (Human))
Huazhong University of Science and Technology
Curated by ChEMBL
Huazhong University of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibitory activity against SSADHMore data for this Ligand-Target Pair
Target4-aminobutyrate aminotransferase, mitochondrial(Homo sapiens (Human))
Huazhong University of Science and Technology
Curated by ChEMBL
Huazhong University of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibitory activity against GABATMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Hinoki Shinyaku Co., Ltd
Curated by ChEMBL
Hinoki Shinyaku Co., Ltd
Curated by ChEMBL
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of mushroom tyrosinase using L-tyrosine as substrate assessed as dopachrome formation preincubated for 10 mins followed by protein additio...More data for this Ligand-Target Pair
TargetDihydropteroate synthase(Bacillus anthracis)
University of Tennessee Health Science Center
Curated by ChEMBL
University of Tennessee Health Science Center
Curated by ChEMBL
Affinity DataKd: 5.62E+3nMAssay Description:Binding affinity to Bacillus anthracis DHPS expressed in Escherichia coli BL21 (DE3) after 30 mins by isothermal titration calorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 35.2nMAssay Description:Inhibition of AChE activity in rat brain homogenate using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Hinoki Shinyaku Co., Ltd
Curated by ChEMBL
Hinoki Shinyaku Co., Ltd
Curated by ChEMBL
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of mushroom tyrosinase using L-tyrosine as substrate assessed as dopachrome formation preincubated for 10 mins followed by protein additio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+5nMAssay Description:Inhibition of recombinant human CYP1B1 expressed in Escherichia coli DH5aplha cells assessed as O-deethylation of ethoxyresorufin in presence of NADP...More data for this Ligand-Target Pair