null
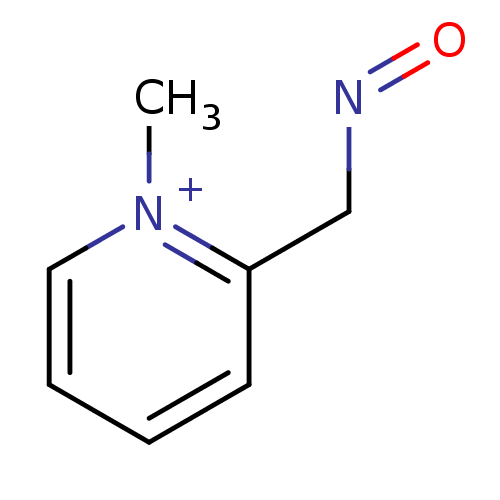
SMILES C[n+]1ccccc1CN=O
InChI Key InChIKey=MHOAUIZFSQGCNM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 29 hits for monomerid = 50011780
Found 29 hits for monomerid = 50011780
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
SRI International
Curated by ChEMBL
SRI International
Curated by ChEMBL
Affinity DataKi: 1.27E+5nMAssay Description:Compound was tested for the competitive inhibition of phosphorylation of Eel acetylcholinesterase (AChE)More data for this Ligand-Target Pair
Affinity DataIC50: 9.96E+5nMAssay Description:Inhibition of human AChE assessed as decrease in enzyme activity incubated for 30 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:In vitro reversible inhibition of eel acetylcholinesterase.More data for this Ligand-Target Pair
Affinity DataKd: 2.15E+5nMAssay Description:Reactivation of methylphosphonothioate VX-inhibited recombinant human AChE measured up to 10 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.19E+6nMAssay Description:Inhibition of hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up to 1 hr by Ellman m...More data for this Ligand-Target Pair
Affinity DataKd: 2.57E+4nMAssay Description:Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ...More data for this Ligand-Target Pair
Affinity DataKd: 3.14E+4nMAssay Description:Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ...More data for this Ligand-Target Pair
Affinity DataKd: 1.78E+4nMAssay Description:Reactivation of VX-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye bas...More data for this Ligand-Target Pair
Affinity DataKd: 3.47E+4nMAssay Description:Reactivation of O-ethylsarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DT...More data for this Ligand-Target Pair
Affinity DataKd: 2.88E+4nMAssay Description:Reactivation of sarin-inhibited human acetylcholinesterase assessed as dissociation constant incubated for 60 mins using ATChI substrate by DTNB dye ...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1/M2/M3/M4/M5(Mus musculus)
SRI International
Curated by ChEMBL
SRI International
Curated by ChEMBL
Affinity DataIC50: 1.70E+5nMAssay Description:Compound was tested for the binding affinity towards muscarinic receptor in mouse brain membraneMore data for this Ligand-Target Pair
Affinity DataKd: 3.14E+4nMAssay Description:Binding affinity to VX-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectropho...More data for this Ligand-Target Pair
Affinity DataKd: 2.57E+4nMAssay Description:Binding affinity to sarin-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectro...More data for this Ligand-Target Pair
Affinity DataIC50: 1.21E+6nMAssay Description:Inhibition of hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectrophotometry based Ellma...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Rattus norvegicus (rat))
Human BioMolecular Research Institute
Curated by ChEMBL
Human BioMolecular Research Institute
Curated by ChEMBL
Affinity DataKd: 2.10E+5nMAssay Description:Binding affinity to AChE in rat brain homogenateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+5nMAssay Description:Reversible inhibition of Human AchEMore data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+5nMAssay Description:Compound was tested for the competitive inhibition of acetylcholinesterase (AChE)More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
SRI International
Curated by ChEMBL
SRI International
Curated by ChEMBL
Affinity DataIC50: 1.85E+5nMAssay Description:Inhibition of eel acetylcholinesterase (AChE) activity by 50% More data for this Ligand-Target Pair
Affinity DataIC50: 3.66E+5nMAssay Description:Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC).More data for this Ligand-Target Pair
Affinity DataIC50: 3.46E+5nMAssay Description:Inhibition of acetylcholinesterase (AChE) in bovine erythrocyte(RBC)More data for this Ligand-Target Pair
Affinity DataIC50: 3.66E+5nMAssay Description:In vitro concentration of compound required for reversibly inhibiting 50% of human anticholinesterase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 8.78E+5nMAssay Description:Inhibition of human recombinant AChE by modified Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.78E+5nMAssay Description:Inhibition of human recombinant AChEMore data for this Ligand-Target Pair
Affinity DataKd: 2.76E+4nMAssay Description:Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKd: 3.16E+6nMAssay Description:Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr...More data for this Ligand-Target Pair
Affinity DataKd: 7.06E+5nMAssay Description:Reactivation of tabun inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKd: 2.81E+4nMAssay Description:Reactivation of VX inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate addi...More data for this Ligand-Target Pair
Affinity DataIC50: 3.66E+5nMAssay Description:Reversible inhibition of acetylcholinesterase(AChE) by 50 % in human erythrocytes More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
SRI International
Curated by ChEMBL
SRI International
Curated by ChEMBL
Affinity DataIC50: 4.00E+5nMAssay Description:In vitro reversible inhibition of eel acetylcholinesterase.More data for this Ligand-Target Pair