null
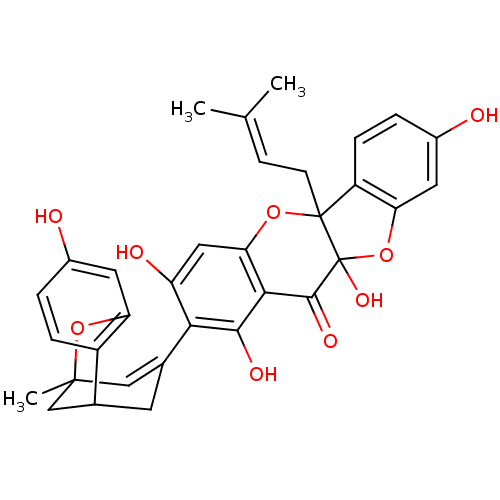
SMILES [#6]\[#6](-[#6])=[#6]\[#6]C12[#8]-c3cc(-[#8])c(-[#6]-4=[#6]C5([#6])[#6]-[#6](-[#6]-4)-c4ccc(-[#8])cc4-[#8]5)c(-[#8])c3-[#6](=O)C1([#8])[#8]-c1cc(-[#8])ccc21
InChI Key InChIKey=GJKQLIDFFREHGO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50377903
Found 2 hits for monomerid = 50377903
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+4nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair