null
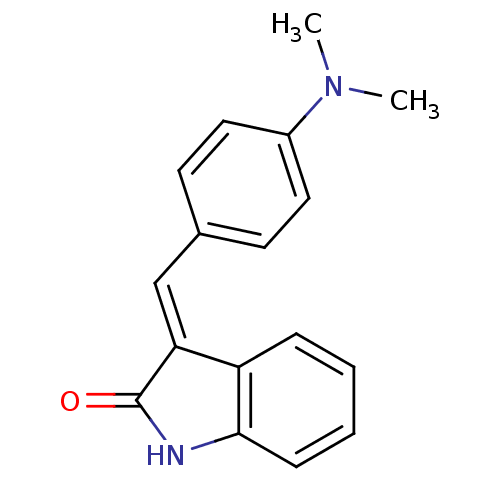
SMILES CN(C)c1ccc(\C=C2\C(=O)Nc3ccccc23)cc1
InChI Key InChIKey=UAKWLVYMKBWHMX-RVDMUPIBSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50111603
Found 7 hits for monomerid = 50111603
TargetPlatelet-derived growth factor receptor beta(Homo sapiens (Human))
Duquesne University of the Holy Ghost
US Patent
Duquesne University of the Holy Ghost
US Patent
Affinity DataIC50: 3.75E+3nMAssay Description:Inhibition of various kinase enzyme.More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Mus musculus)
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 794nMAssay Description:Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1)More data for this Ligand-Target Pair
Affinity DataIC50: 2.75E+4nMAssay Description:Inhibition of EPH receptor B2 using ELISAMore data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor beta(Homo sapiens (Human))
Duquesne University of the Holy Ghost
US Patent
Duquesne University of the Holy Ghost
US Patent
Affinity DataIC50: 3.75E+3nMAssay Description:Table 9: Compounds 1 and 2, Section D., (see FIG. 4, bottom row, far right column) each inhibit VEGFR-2 and PDGFR-β for antiangiogenic effects a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity against beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibitory activity against chymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataKd: 1.95E+4nMAssay Description:Equilibrium binding constant for EPH receptor B2More data for this Ligand-Target Pair