null
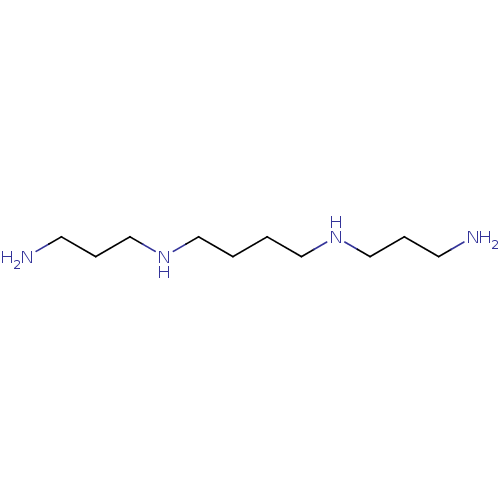
SMILES NCCCNCCCCNCCCN
InChI Key InChIKey=PFNFFQXMRSDOHW-UHFFFAOYSA-N
PDB links: 210 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 30 hits for monomerid = 79403
Found 30 hits for monomerid = 79403
Affinity DataKi: 10nMAssay Description:Inhibition of human carbonic anhydrase 4 by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 710nMAssay Description:Inhibition of human carbonic anhydrase 7 by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
TargetCarbonic anhydrase 5B, mitochondrial(Homo sapiens (Human))
Universita degli Studi di Firenze
Curated by ChEMBL
Universita degli Studi di Firenze
Curated by ChEMBL
Affinity DataKi: 830nMAssay Description:Inhibition of human carbonic anhydrase 5B by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
TargetCarbonic anhydrase 5A, mitochondrial(Homo sapiens (Human))
Universita degli Studi di Firenze
Curated by ChEMBL
Universita degli Studi di Firenze
Curated by ChEMBL
Affinity DataKi: 840nMAssay Description:Inhibition of human carbonic anhydrase 5A by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 860nMAssay Description:Inhibition of human carbonic anhydrase 14 by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 990nMAssay Description:Inhibition of human carbonic anhydrase 6 by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1(RAT)
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
TargetIonotropic glutamate receptor subunit Delta2(Xenopus)
State University of New York
Curated by PDSP Ki Database
State University of New York
Curated by PDSP Ki Database
Affinity DataKi: 1.33E+4nMAssay Description:Inhibition of human carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.33E+4nMAssay Description:Inhibition of human carbonic anhydrase 9 by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.26E+4nMAssay Description:Inhibition of mouse carbonic anhydrase 13 by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.76E+4nMAssay Description:Inhibition of human carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.76E+4nMAssay Description:Inhibition of human carbonic anhydrase 12 by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 7.40E+4nMAssay Description:Inhibition of mouse carbonic anhydrase 15 by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 8.40E+4nMAssay Description:Inhibition of human carbonic anhydrase 2 by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 8.40E+4nMAssay Description:Inhibition of human carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.67E+5nMAssay Description:Inhibition of human carbonic anhydrase 3 by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.31E+5nMAssay Description:Inhibition of human carbonic anhydrase 1 by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.31E+5nMAssay Description:Inhibition of human carbonic anhydrase 1 preincubated for 15 mins by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
University of Bristol
Curated by ChEMBL
University of Bristol
Curated by ChEMBL
Affinity DataEC50: 1.25E+5nMAssay Description:Positive allosteric modulation of GluN2B receptor (unknown origin) in hippocampal neurons assessed as increase in glycine-induced channel current by ...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
University of Bristol
Curated by ChEMBL
University of Bristol
Curated by ChEMBL
Affinity DataEC50: 8.10E+4nMAssay Description:Positive allosteric modulation of GluN2B receptor in rat spinal cord neurons assessed as increase in glycine-induced channel current by two electrode...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
University of Bristol
Curated by ChEMBL
University of Bristol
Curated by ChEMBL
Affinity DataEC50: 1.27E+5nMAssay Description:Positive allosteric modulation of GluN2B receptor (unknown origin) expressed in xenopus laevis oocytes assessed as increase in glycine-induced channe...More data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A/1B(Bos taurus)
Universit£ Rennes 1
Curated by ChEMBL
Universit£ Rennes 1
Curated by ChEMBL
Affinity DataIC50: 5.00E+5nMAssay Description:Inhibition of bovine calmodulin-activated cAMP dependent phosphodiesteraseMore data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Department of Pharmacy and Biotechnology, Alma Mater Studiorum-University of Bologna, Via Belmeloro 6, 40126 Bologna, Italy; Laboratory of Molecular Modeling and Drug Discovery, Istituto Italiano di
Curated by ChEMBL
Department of Pharmacy and Biotechnology, Alma Mater Studiorum-University of Bologna, Via Belmeloro 6, 40126 Bologna, Italy; Laboratory of Molecular Modeling and Drug Discovery, Istituto Italiano di
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of recombinant human topoisomerase-2alpha expressed in Saccharomyces cerevisiae JEL1 harboring topoisomerase1 deletion mutant assessed as ...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Rattus norvegicus (Rat))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataEC50: 1.00E+5nMAssay Description:Positive allosteric modulation of recombinant rat GluN1/GluN2B receptor expressed in xenopus laevis oocyte assessed as potentiation of glycine-induce...More data for this Ligand-Target Pair
Affinity DataEC50: 8.50E+3nMAssay Description:Activation of procaspase 2 after 24 hrsMore data for this Ligand-Target Pair
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.43E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
In DepthDetails
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.56E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
TargetBcl-2-related protein A1(Mus musculus (Mouse))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 2.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)