null
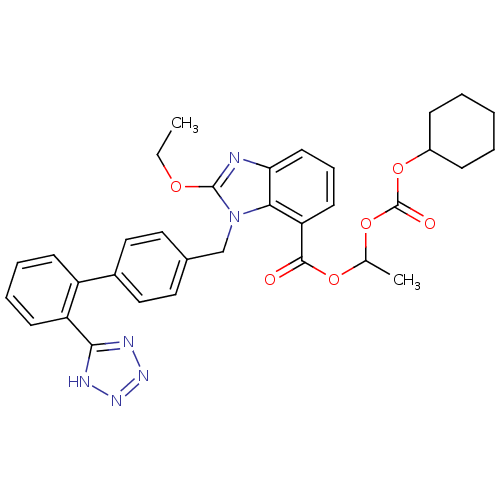
SMILES CCOc1nc2cccc(C(=O)OC(C)OC(=O)OC3CCCCC3)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1
InChI Key InChIKey=GHOSNRCGJFBJIB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 50318907
Found 15 hits for monomerid = 50318907
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
KU Leuven
Curated by ChEMBL
KU Leuven
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Ki values for sodium fluorescein (10 uM) uptake in OATP1B1-transfected CHO cellsMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B3(Homo sapiens (Human))
KU Leuven
Curated by ChEMBL
KU Leuven
Curated by ChEMBL
Affinity DataKi: 1.90E+3nMAssay Description:Ki values for sodium fluorescein (10 uM) uptake in OATP1B3-transfected CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Antagonist activity at angiotensin AT1 receptor in rabbit aortic strip assessed as inhibition of angiotensin 2-induced contractile responseMore data for this Ligand-Target Pair
TargetDNA polymerase iota(Homo sapiens (Human))
University of Connecticut Health Center
Curated by ChEMBL
University of Connecticut Health Center
Curated by ChEMBL
Affinity DataIC50: 6.20E+3nMAssay Description:Inhibition of human DNA polymerase iota preincubated for 15 mins followed by replicating non-damaged DNA substrate addition measured after 30 mins in...More data for this Ligand-Target Pair
TargetDNA polymerase eta(Homo sapiens (Human))
University of Connecticut Health Center
Curated by ChEMBL
University of Connecticut Health Center
Curated by ChEMBL
Affinity DataIC50: 1.12E+4nMAssay Description:Inhibition of human DNA polymerase eta (1 to 437 residues) preincubated for 15 mins followed by replicating non-damaged DNA substrate addition measur...More data for this Ligand-Target Pair
TargetDNA polymerase kappa(Homo sapiens (Human))
University of Connecticut Health Center
Curated by ChEMBL
University of Connecticut Health Center
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of human DNA polymerase kappa (19 to 526 residues)-mediated TLS past acrolein derived ring-opened reduced form of gamma-HOPdG lesions prei...More data for this Ligand-Target Pair
TargetDNA polymerase kappa(Homo sapiens (Human))
University of Connecticut Health Center
Curated by ChEMBL
University of Connecticut Health Center
Curated by ChEMBL
Affinity DataIC50: 9.20E+3nMAssay Description:Inhibition of human DNA polymerase kappa (19 to 526 residues) preincubated for 15 mins followed by replicating non-damaged DNA substrate addition mea...More data for this Ligand-Target Pair
Affinity DataIC50: 1.68E+5nMAssay Description:Inhibition of Trypanosoma cruzi cruzaine preincubated for 5 mins before substrate addition by fluorescence assay in presence of 0.01% Triton X-100More data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+4nMAssay Description:Inhibition of Trypanosoma cruzi cruzaine preincubated for 5 mins before substrate addition by fluorescence assay in absence of Triton X-100More data for this Ligand-Target Pair
TargetNEDD8-activating enzyme E1 catalytic subunit(Homo sapiens)
Shanghai University of Traditional Chinese Medicine
Curated by ChEMBL
Shanghai University of Traditional Chinese Medicine
Curated by ChEMBL
Affinity DataKd: 1.95E+5nMAssay Description:Binding affinity to biotin-labeled GST-tagged NAE (unknown origin) incubated for 1 hr by SPR assayMore data for this Ligand-Target Pair
Affinity DataEC50: 4.20E+3nMAssay Description:Agonist activity at human PPARgammaDEF receptor expressed in african green monkey COS7 cells transfected with pGal5-TK-pGL3/pRenilla-CMV assessed as ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.64E+4nMAssay Description:Inhibition of NAE (unknown origin)-mediated neddylation assessed as suppression of cullin1-Nedd8 adduct formation preincubated for 10 mins followed b...More data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
KU Leuven
Curated by ChEMBL
KU Leuven
Curated by ChEMBL
Affinity DataIC50: 724nMAssay Description:pIC50 values for sodium fluorescein (10 uM) uptake in OATP1B1-transfected CHO cellsMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B3(Homo sapiens (Human))
KU Leuven
Curated by ChEMBL
KU Leuven
Curated by ChEMBL
Affinity DataIC50: 2.46E+3nMAssay Description:pIC50 values for sodium fluorescein (10 uM) uptake in OATP1B3-transfected CHO cellsMore data for this Ligand-Target Pair