null
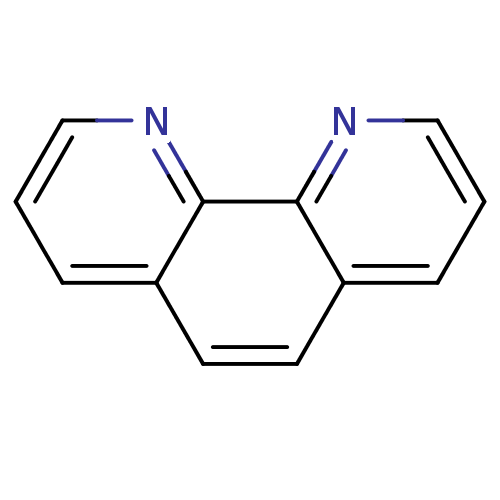
SMILES c1cnc2c(c1)ccc1cccnc21
InChI Key InChIKey=DGEZNRSVGBDHLK-UHFFFAOYSA-N
PDB links: 10 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 33 hits for monomerid = 50092158
Found 33 hits for monomerid = 50092158
TargetGalanin receptor type 2(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 4.20E+4nMAssay Description:Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Florida Research Institute, TSRI Assay...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Table 10: The efficacy of MMP-2 inhibition was as follows: compound 6>compound 7>compound 8. Although compound 6 showed similar efficacy as curcumin ...More data for this Ligand-Target Pair
TargetRecBCD enzyme subunit RecD(Escherichia coli str. K-12 substr. MG1655)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 3.05E+4nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa...More data for this Ligand-Target Pair
TargetNeutrophil collagenase(Homo sapiens (Human))
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
Affinity DataIC50: 1.00E+4nMT: 2°CAssay Description:It has been observed that 50 and 100 μM concentrations of curcumin decreased TNFα production by endotoxin-stimulated human monocytes (HMs) ...More data for this Ligand-Target Pair
TargetNeutrophil collagenase(Homo sapiens (Human))
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
Affinity DataIC50: 3.50E+4nMT: 2°CAssay Description:It has been observed that 50 and 100 μM concentrations of curcumin decreased TNFα production by endotoxin-stimulated human monocytes (HMs) ...More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
Affinity DataIC50: 9.00E+3nMT: 2°CAssay Description:It has been observed that 50 and 100 μM concentrations of curcumin decreased TNFα production by endotoxin-stimulated human monocytes (HMs) ...More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
Affinity DataIC50: 7.00E+4nMT: 2°CAssay Description:It has been observed that 50 and 100 μM concentrations of curcumin decreased TNFα production by endotoxin-stimulated human monocytes (HMs) ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMT: 2°CAssay Description:It has been observed that 50 and 100 μM concentrations of curcumin decreased TNFα production by endotoxin-stimulated human monocytes (HMs) ...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Kumamoto University
Curated by ChEMBL
Kumamoto University
Curated by ChEMBL
Affinity DataIC50: 4.00E+6nMAssay Description:Inhibition of [3H]-farnesyl pyrophosphate binding to human farnesyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7.30E+4nMAssay Description:Inhibition of Pseudomonas aeruginosa VIM-1 beta-lactamase after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.93E+4nMAssay Description:Inhibition of Pseudomonas aeruginosa VIM-13 beta-lactamase after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+4nMAssay Description:Displacement of [125I]-CCL3 from human CCR1 transfected in COS7 cells coexpressing chimeric Gqi4myr after 3 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 5.01E+3nMAssay Description:Allosteric modulation at human CCR5 transfected in COS7 cells coexpressing chimeric Gqi4myr assessed as [3H]IP3 turnover by liquid scintillation coun...More data for this Ligand-Target Pair
Affinity DataEC50: 6.31E+3nMAssay Description:Allosteric modulation at human CCR1 transfected in COS7 cells coexpressing chimeric Gqi4myr assessed as {3H]IP3 turnover by liquid scintillation coun...More data for this Ligand-Target Pair
Affinity DataEC50: 3.90E+3nMAssay Description:Allosteric modulation at human CCR8 transfected in COS7 cells assessed as [3H]IP3 turnover by liquid scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5.30E+4nMAssay Description:Displacement of [125I]-CCL3 from human CCR5 transfected in COS7 cells coexpressing chimeric Gqi4myr after 3 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 5.90E+3nMAssay Description:Allosteric modulation at human CCR1 transfected in COS7 cells coexpressing chimeric Gqi4myr assessed as {3H]IP3 turnover by liquid scintillation coun...More data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Displacement of [125I]-CCL3 from human CCR5 transfected in COS7 cells coexpressing chimeric Gqi4myr after 3 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.94E+4nMAssay Description:Displacement of [125I]-CCL3 from human CCR1 transfected in COS7 cells coexpressing chimeric Gqi4myr after 3 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 4.90E+3nMAssay Description:Allosteric modulation at human CCR5 transfected in COS7 cells coexpressing chimeric Gqi4myr assessed as [3H]IP3 turnover by liquid scintillation coun...More data for this Ligand-Target Pair
Affinity DataEC50: 3.98E+3nMAssay Description:Allosteric modulation at human CCR8 transfected in COS7 cells assessed as [3H]IP3 turnover by liquid scintillation counting analysisMore data for this Ligand-Target Pair
TargetSnake venom metalloproteinase neuwiedase(Bothrops pauloensis)
Instituto de Ci£ncias Exatas
Curated by ChEMBL
Instituto de Ci£ncias Exatas
Curated by ChEMBL
Affinity DataIC50: 4.70E+5nMAssay Description:Inhibition of Bothrops pauloensis MP1 assessed as reduction in azocaseinolytic activity after 30 minsMore data for this Ligand-Target Pair
TargetThermolysin(Bacillus thermoproteolyticus)
Osaka Organic Chemical Industry, Ltd
Curated by ChEMBL
Osaka Organic Chemical Industry, Ltd
Curated by ChEMBL
Affinity DataIC50: 3.60E+5nMAssay Description:Inhibition of Bacillus thermoproteolyticus thermolysin after 15 minMore data for this Ligand-Target Pair
TargetCollagenase ColG(Clostridium histolyticum)
Osaka Organic Chemical Industry, Ltd
Curated by ChEMBL
Osaka Organic Chemical Industry, Ltd
Curated by ChEMBL
Affinity DataIC50: 1.85E+5nMAssay Description:Inhibition of Clostridium histolyticum collagenase after 15 minMore data for this Ligand-Target Pair
Affinity DataIC50: 4.22E+5nMAssay Description:Inhibition of Bos taurus (bovine) pancrease carboxypeptidase A after 15 minMore data for this Ligand-Target Pair
TargetNeutrophil collagenase(Homo sapiens (Human))
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
Affinity DataIC50: 2.25E+4nMAssay Description:It has been observed that 50 and 100 μM concentrations of curcumin decreased TNFα production by endotoxin-stimulated human monocytes (HMs) ...More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
Affinity DataIC50: 9.00E+3nMAssay Description:It has been observed that 50 and 100 μM concentrations of curcumin decreased TNFα production by endotoxin-stimulated human monocytes (HMs) ...More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
Affinity DataIC50: 7.00E+4nMAssay Description:It has been observed that 50 and 100 μM concentrations of curcumin decreased TNFα production by endotoxin-stimulated human monocytes (HMs) ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:It has been observed that 50 and 100 μM concentrations of curcumin decreased TNFα production by endotoxin-stimulated human monocytes (HMs) ...More data for this Ligand-Target Pair
TargetNeutrophil collagenase(Homo sapiens (Human))
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
Affinity DataIC50: 2.25E+4nMAssay Description:Table 1 shows the IC50 of curcumin, compound 1, and compound 2, compared to a standard Zn++ binding & MMPI (matrix metalloproteinase inhibitor), 1,10...More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-9(Homo sapiens (Human))
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
Affinity DataIC50: 9.00E+3nMAssay Description:Table 1 shows the IC50 of curcumin, compound 1, and compound 2, compared to a standard Zn++ binding & MMPI (matrix metalloproteinase inhibitor), 1,10...More data for this Ligand-Target Pair
Target72 kDa type IV collagenase(Homo sapiens (Human))
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
The Research Foundation of State University of New York; Chem-Master International, Inc.
US Patent
Affinity DataIC50: 7.00E+4nMAssay Description:Table 10: The efficacy of MMP-2 inhibition was as follows: compound 6>compound 7>compound 8. Although compound 6 showed similar efficacy as curcumin ...More data for this Ligand-Target Pair
TargetGalanin receptor type 2(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 4.20E+4nMAssay Description:Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Research Institute (TSRI) Assay Provid...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)