null
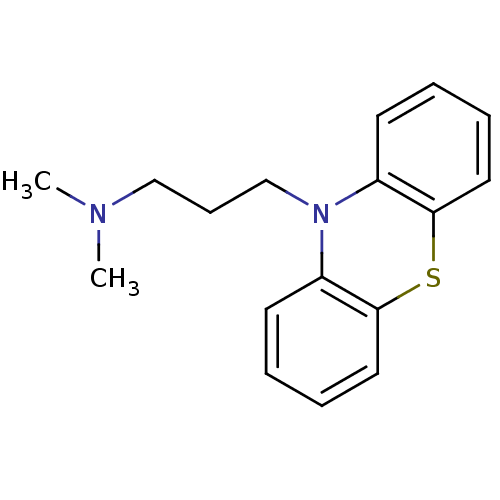
SMILES CN(C)CCCN1c2ccccc2Sc2ccccc12
InChI Key InChIKey=ZGUGWUXLJSTTMA-UHFFFAOYSA-N
PDB links: 2 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 32 hits for monomerid = 67545
Found 32 hits for monomerid = 67545
TargetHistamine H1 receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetHistamine H1 receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetD(2) dopamine receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetD(2) dopamine receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetD(2) dopamine receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetAlpha-2A adrenergic receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
TargetAlpha-2A adrenergic receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1A/1B/1D/2C(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Affinity DataKi: 5.91E+4nMAssay Description:Inhibitory activity against recombinant Trypanosoma cruzi (T. cruzi) Trypanothione reductase (linear competitive type)More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Mucosa-associated lymphoid tissue lymphoma translocation protein 1(Homo sapiens (Human))
HELMHOLTZ ZENTRUM MUNCHEN, DEUTSCHES FORSCHUNGSZENTRUM FUR GESUNDHEIT UND UMWELT (GMBH)
US Patent
HELMHOLTZ ZENTRUM MUNCHEN, DEUTSCHES FORSCHUNGSZENTRUM FUR GESUNDHEIT UND UMWELT (GMBH)
US Patent
Affinity DataIC50: 5.80E+3nMT: 2°CAssay Description:To screen for small molecular weight compounds that can inhibit MALT1 protease activity, recombinant GSTMALT1 was purified from E. coli to establish ...More data for this Ligand-Target Pair
In DepthDetails
TargetMajor prion protein(Homo sapiens (Human))
Institut f£r Molekularbiologie und Biophysik
Curated by ChEMBL
Institut f£r Molekularbiologie und Biophysik
Curated by ChEMBL
Affinity DataEC50: 5.00E+3nMAssay Description:Half maximal inhibition of Prion protein PrPsc formation was assayed in ScN2a cellsMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Mayo Clinic and Foundation
Curated by PDSP Ki Database
Affinity DataIC50: 2.45E+3nMAssay Description:Antagonist activity at H1 receptor in human HeLa cells assessed as inhibition of histamine-induced Ca2+ release by using fura-2AM-based fluorescence ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+3nMAssay Description:Displacement of [3H]mepyramine from histamine H1 receptor in Sprague-Dawley rat brain membrane after 2 hr by scintillation countingMore data for this Ligand-Target Pair
TargetNADPH oxidase 1(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 1.70E+4nMAssay Description:Data Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institut...More data for this Ligand-Target Pair
Affinity DataIC50: 1.72E+4nMAssay Description:Inhibition of 4-(4-(dimethylamino)styryl)-N-methylpyridinium uptake at human OCT1 expressed in HEK293 cells by confocal microscopyMore data for this Ligand-Target Pair
TargetPleiotropic ABC efflux transporter of multiple drugs(Saccharomyces cerevisiae S288c)
Wroclaw Medical University
Curated by ChEMBL
Wroclaw Medical University
Curated by ChEMBL
Affinity DataIC50: 1.13E+4nMAssay Description:Inhibition of Pdr5p-mediated rhodamine 6G transport in Saccharomyces cerevisiae MKPDR5h plasma membrane by spectrofluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Compound was tested for inhibition of [3H]spiperone binding in membrane preparations obtained from calf caudate.More data for this Ligand-Target Pair
Affinity DataIC50: 1.04E+3nMAssay Description:Compound was tested for inhibition of [3H]spiperone binding in membrane preparations obtained from rat corpus striatum.More data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of binding of Batrachotoxinin [3H]BTX-B to high affinity sites on voltage dependent sodium channels in a vesicular preparation from guinea...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)