null
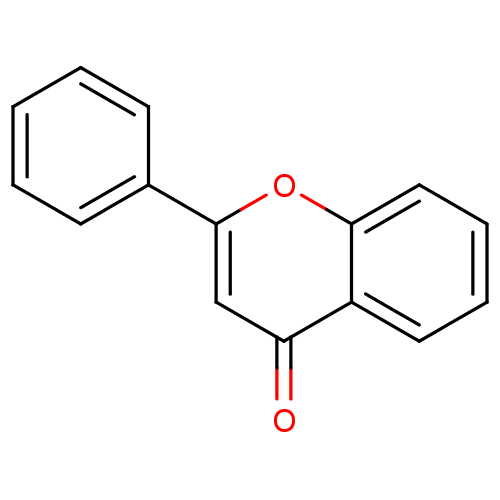
SMILES O=c1cc(oc2ccccc12)-c1ccccc1
InChI Key InChIKey=VHBFFQKBGNRLFZ-UHFFFAOYSA-N
PDB links: 2 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 37 hits for monomerid = 50028962
Found 37 hits for monomerid = 50028962
Affinity DataKi: 1.06E+3nMAssay Description:The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =...More data for this Ligand-Target Pair
Affinity DataKi: 1.88E+3nMAssay Description:Inhibition of cytosolic human carbonic anhydrase 1 preincubated for 12 hrs by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.89E+3nMAssay Description:Inhibition of cytosolic human carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =...More data for this Ligand-Target Pair
Affinity DataKi: 3.28E+3nMAssay Description:Displacement of specific [3H]-PIA binding from adenosine A1 receptor in rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 3.28E+3nMAssay Description:Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H]-PIA displacement.More data for this Ligand-Target Pair
Affinity DataKi: 3.31E+3nMAssay Description:Ability to displace [3H]N6-phenylisopropyladenosine binding from adenosine A1 receptor.Checked by AuthorMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Rattus norvegicus (rat))
National Institute of Diabetes
Curated by ChEMBL
National Institute of Diabetes
Curated by ChEMBL
Affinity DataKi: 3.45E+3nMAssay Description:Affinity at Adenosine A2A receptor in rat striatal membranes by [3H]- CGS 21680 displacement.More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Rattus norvegicus (rat))
National Institute of Diabetes
Curated by ChEMBL
National Institute of Diabetes
Curated by ChEMBL
Affinity DataKi: 3.47E+3nMAssay Description:Ability to displace [3H]-CGS- 21680 binding from adenosine A2A receptor.Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 4.13E+3nMAssay Description:Inhibition of transmembrane tumor-associated human carbonic anhydrase 9 preincubated for 15 mins by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.22E+3nMAssay Description:Inhibition of transmembrane tumor-associated human carbonic anhydrase 9 preincubated for 12 hrs by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
TargetCarbonic anhydrase 12(Homo sapiens (Human))
Universit£ degli Studi di Cagliari
Curated by ChEMBL
Universit£ degli Studi di Cagliari
Curated by ChEMBL
Affinity DataKi: 7.22E+3nMAssay Description:Inhibition of transmembrane tumor-associated human carbonic anhydrase 12 preincubated for 15 mins by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
TargetCarbonic anhydrase 12(Homo sapiens (Human))
Universit£ degli Studi di Cagliari
Curated by ChEMBL
Universit£ degli Studi di Cagliari
Curated by ChEMBL
Affinity DataKi: 7.33E+3nMAssay Description:Inhibition of transmembrane tumor-associated human carbonic anhydrase 12 preincubated for 12 hrs by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.71E+3nMAssay Description:Inhibition of cytosolic human carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.80E+3nMAssay Description:Inhibition of cytosolic human carbonic anhydrase 2 preincubated for 12 hrs by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.69E+4nMAssay Description:Binding affinity against human adenosine A3 receptor in HEK293 cells using [125I]-AB-MECA 21680 radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 1.69E+4nMAssay Description:Displacement of [125I]-AB-MECA binding to human Adenosine A3 receptor expressed in HEK-293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+4nMAssay Description:Ability to displace [125I]-AB-MECA binding from adenosine A3 receptor.Checked by AuthorMore data for this Ligand-Target Pair
TargetCytochrome P450 1B1(Homo sapiens (Human))
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of human CYP1B1 expressed in Escherichia coli DH5alpha coexpressing human NADPH P450 reductase using 7-ethoxyresorufin as substrate in pre...More data for this Ligand-Target Pair
Affinity DataIC50: 7.76E+4nMAssay Description:Inhibitory concentration against recombinant rat androgen receptor expressed in Escherichia coli using [3H]methyltrienolone (R 1881)More data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (fumarate)(Saccharomyces cerevisiae)
Harvard School of Public Health
Curated by ChEMBL
Harvard School of Public Health
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Saccharomyces cerevisiae DHODMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Plasmodium falciparum)
Harvard School of Public Health
Curated by ChEMBL
Harvard School of Public Health
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Plasmodium falciparum DHODMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human placental microsome CYP19More data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase tankyrase-1(Homo sapiens (Human))
University of Oulu
Curated by ChEMBL
University of Oulu
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:Inhibition of human 6XHis-tagged TNKS1 SAM-ART domain (1030 to 1317 amino acid residues) by fluorescence assayMore data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase tankyrase-2(Homo sapiens (Human))
University of Oulu
Curated by ChEMBL
University of Oulu
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of human 6XHis-tagged TNKS2 ART domain (946 to 1161 amino acid residues) expressed in Escherichia coli Rosetta2 (DE3) by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human recombinant ARTD1 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKd: 3.39E+4nMAssay Description:Binding affinity to ABCB1 nucleotide binding domain 2More data for this Ligand-Target Pair
Affinity DataIC50: 170nMAssay Description:Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ...More data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Homo sapiens (Human))
University of Bonn
Curated by ChEMBL
University of Bonn
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of BCRP expressed in MDCK cells using Hoechst 33342 stainingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+5nMAssay Description:Inhibition of human recombinant calmodulin assessed as inhibition of calmodulin-sensitive cAMP phosphodiesterase activation after 15 mins by spectrop...More data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMAssay Description:Antagonist activity at androgen receptor in human MDA-kb2 cells assessed as inhibition of DHT-induced luciferase activity by luciferase reporter gene...More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of aromatase (unknown origin)More data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Homo sapiens (Human))
University of Bonn
Curated by ChEMBL
University of Bonn
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of BCRP expressed in MDCK cells using Hoechst 33342 stainingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.24E+5nMAssay Description:Inhibition of EGFR in human A431 cellsMore data for this Ligand-Target Pair
TargetDNA-dependent protein kinase catalytic subunit(Homo sapiens (Human))
The University
Curated by ChEMBL
The University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of DNA dependent protein kinase isolated from HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.93E+5nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of aromatase (unknown origin)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)