null
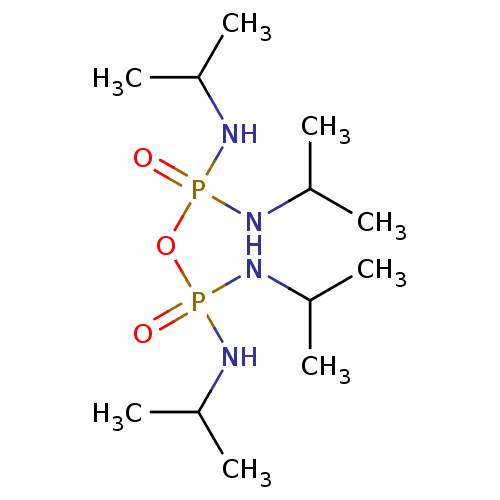
SMILES CC(C)NP(=O)(NC(C)C)OP(=O)(NC(C)C)NC(C)C
InChI Key InChIKey=IOIMDJXKIMCMIG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 10625
Found 7 hits for monomerid = 10625
Affinity DataIC50: 3.40E+5nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 980nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 134nMpH: 8.0Assay Description:The reaction mixture was consisted of 100 ÁL of the total volume. To the solution of PBS (pH 8.0, 30 ÁL) consisted of KH2PO4 and K2HPO4 in 96-well pl...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Institute for Natural Products Applications and Research Technologies
Curated by ChEMBL
Institute for Natural Products Applications and Research Technologies
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of electric eel AChE incubated for 15 mins by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Institute for Natural Products Applications and Research Technologies
Curated by ChEMBL
Institute for Natural Products Applications and Research Technologies
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of electric eel AchE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Institute for Natural Products Applications and Research Technologies
Curated by ChEMBL
Institute for Natural Products Applications and Research Technologies
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of electric eel AChE incubated for 15 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 733nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair