null
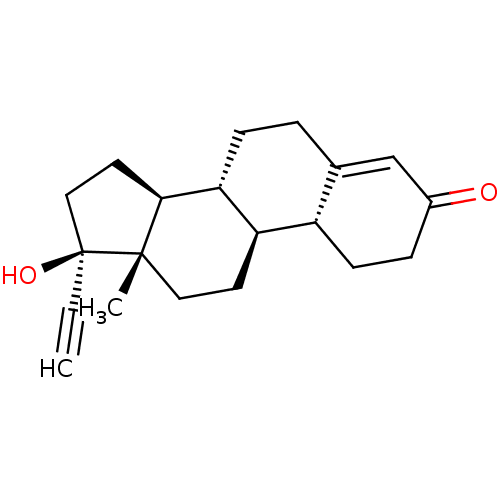
SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@H]34)[C@@H]1CC[C@@]2(O)C#C
InChI Key InChIKey=VIKNJXKGJWUCNN-XGXHKTLJSA-N
PDB links: 2 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50148732
Found 9 hits for monomerid = 50148732
Affinity DataKi: 1.90nMAssay Description:The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Binding affinity against Progesterone receptor in human TE85 osteosarcoma cells was determined using (Z)-[125I]-17-alpha-(2-iodovinyl)-19-nor-testost...More data for this Ligand-Target Pair
Affinity DataEC50: 2.20nMAssay Description:Agonistic activity was measured for modulation of hPR-B (human progesterone receptor) in co-transfected CV-1 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+4nMAssay Description:Ability to inhibit HMG-CoA reductase (HMGR) by cholesterol synthesis inhibition screen (CSI) in ratsMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibitory concentration against recombinant rat androgen receptor expressed in Escherichia coli using [3H]methyltrienolone (R 1881)More data for this Ligand-Target Pair
Affinity DataKd: 0.630nMAssay Description:Equilibrium dissociation constant for rat uterine estrogen receptor binding [3H]estradiolMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+4nMAssay Description:Inhibition of human BSEP expressed in fall armyworm sf9 cell plasma membrane vesicles assessed as reduction in vesicle-associated [3H]-taurocholate t...More data for this Ligand-Target Pair
Affinity DataKd: 0.400nMAssay Description:Dissociation constant for progesterone receptorMore data for this Ligand-Target Pair
TargetSex hormone-binding globulin(Homo sapiens (Human))
University of British Columbia
Curated by ChEMBL
University of British Columbia
Curated by ChEMBL
Affinity DataKd: 11nMAssay Description:Displacement of [3H]5alpha dihydrotestosterone from human sex hormone binding globulinMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)