null
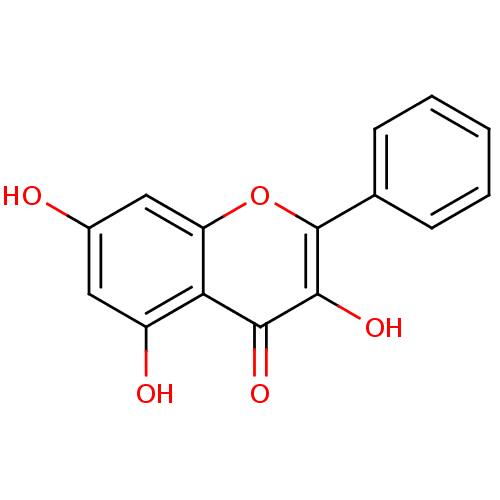
SMILES Oc1cc(O)c2c(c1)oc(-c1ccccc1)c(O)c2=O
InChI Key InChIKey=VCCRNZQBSJXYJD-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 44 hits for monomerid = 50049391
Found 44 hits for monomerid = 50049391
TargetCarbonic anhydrase 7(Homo sapiens (Human))
Aristotle University of Thessaloniki
Curated by ChEMBL
Aristotle University of Thessaloniki
Curated by ChEMBL
Affinity DataKi: 25nM ΔG°: -10.4kcal/moleT: 2°CAssay Description:Inhibition of human recombinant carbonic anhydrase 7 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 12(Homo sapiens (Human))
Aristotle University of Thessaloniki
Curated by ChEMBL
Aristotle University of Thessaloniki
Curated by ChEMBL
Affinity DataKi: 42nM ΔG°: -10.1kcal/moleT: 2°CAssay Description:Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration ass...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 4(Homo sapiens (Human))
Aristotle University of Thessaloniki
Curated by ChEMBL
Aristotle University of Thessaloniki
Curated by ChEMBL
Affinity DataKi: 568nM ΔG°: -8.51kcal/moleT: 2°CAssay Description:Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa...More data for this Ligand-Target Pair
Affinity DataKi: 589nMAssay Description:Ability to displace [125I]-AB-MECA binding from adenosine A3 receptor.Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 863nMAssay Description:Displacement of specific [3H]-PIA binding from adenosine A1 receptor in rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 863nMAssay Description:Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H]-PIA displacement.More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Rattus norvegicus (rat))
National Institute of Diabetes
Curated by ChEMBL
National Institute of Diabetes
Curated by ChEMBL
Affinity DataKi: 966nMAssay Description:Affinity at Adenosine A2A receptor in rat striatal membranes by [3H]- CGS 21680 displacement.More data for this Ligand-Target Pair
Affinity DataKi: 3.15E+3nMAssay Description:Binding affinity against human adenosine A3 receptor in HEK293 cells using [125I]-AB-MECA 21680 radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 3.15E+3nMAssay Description:Displacement of [125I]-AB-MECA binding to human Adenosine A3 receptor expressed in HEK-293 cellsMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute for Medical Research and Occupational Health
Curated by ChEMBL
Institute for Medical Research and Occupational Health
Curated by ChEMBL
Affinity DataKi: 6.90E+3nMAssay Description:Inhibition of human plasma BChE by Ellman's methodMore data for this Ligand-Target Pair
TargetCarbonic anhydrase 1(Homo sapiens (Human))
Aristotle University of Thessaloniki
Curated by ChEMBL
Aristotle University of Thessaloniki
Curated by ChEMBL
Affinity DataKi: >1.00E+4nM ΔG°: >-6.82kcal/moleT: 2°CAssay Description:Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 2(Homo sapiens (Human))
Aristotle University of Thessaloniki
Curated by ChEMBL
Aristotle University of Thessaloniki
Curated by ChEMBL
Affinity DataKi: >1.00E+4nM ΔG°: >-6.82kcal/moleT: 2°CAssay Description:Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa...More data for this Ligand-Target Pair
Affinity DataKi: 1.67E+4nMAssay Description:Binding affinity for HA-tagged wild type human Adenosine A2A receptor (WT) using [3H]CGS-21680 as radioligand expressed in COS-7 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.81E+4nMAssay Description:Binding affinity for HA-tagged mutant human Adenosine A2A receptor (V84L), using [3H]CGS-21680 as radioligand expressed in COS-7 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.30E+4nMAssay Description:Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cellsMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute for Medical Research and Occupational Health
Curated by ChEMBL
Institute for Medical Research and Occupational Health
Curated by ChEMBL
Affinity DataKi: 8.56E+4nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+5nMAssay Description:Inhibition of human aromatase expressed in CHO cellsMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (fumarate)(Leishmania major)
University of S£o Paulo
Curated by ChEMBL
University of S£o Paulo
Curated by ChEMBL
Affinity DataIC50: 4.81E+5nMAssay Description:Inhibition of recombinant oligo-histidine-tagged Leishmania major DHODH expressed in Escherichia coli BL21(DE3) cells using DHO as substrate measured...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute for Medical Research and Occupational Health
Curated by ChEMBL
Institute for Medical Research and Occupational Health
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of AChE (unknown origin)More data for this Ligand-Target Pair
TargetShort transient receptor potential channel 5(Homo sapiens (Human))
University of Nebraska Medical Center
Curated by ChEMBL
University of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:Inhibition of human TRPC5 expressed in HEK293 cells assessed as reduction in gadolinium-induced calcium entry after 30 mins by fluo-4 dye based fluor...More data for this Ligand-Target Pair
TargetSarcoplasmic/endoplasmic reticulum calcium ATPase 1(Oryctolagus cuniculus)
University of Chemistry and Technology Prague
Curated by ChEMBL
University of Chemistry and Technology Prague
Curated by ChEMBL
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibition of rabbit skeletal muscle microsomes SERCA1a preincubated for 10 mins followed by addition of ATP and measured after 40 minsMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase SETD7(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of human SET7 overexpressed in Escherichia coli BL21 (DE3) cells preincubated for 15 mins followed by addition of SAM as substrate and bio...More data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (fumarate)(Leishmania major)
University of S£o Paulo
Curated by ChEMBL
University of S£o Paulo
Curated by ChEMBL
Affinity DataIC50: 5.01E+5nMAssay Description:Inhibition of recombinant oligo-histidine-tagged Leishmania major DHODH expressed in Escherichia coli BL21(DE3) cells using DHO as substrate measured...More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of recombinant human MAO-A assessed as reduction in 4-hydroxyquinoline formation using kynuramine as substrate after 20 mins by fluorometr...More data for this Ligand-Target Pair
Affinity DataIC50: 3.65E+3nMAssay Description:Inhibition of recombinant human MAO-B assessed as reduction in 4-hydroxyquinoline formation using kynuramine as substrate after 20 mins by fluorometr...More data for this Ligand-Target Pair
TargetCytochrome P450 1B1(Homo sapiens (Human))
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human CYP1B1 expressed in Escherichia coli DH5alpha coexpressing human NADPH P450 reductase using 7-ethoxyresorufin as substrate in pre...More data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+6nMAssay Description:In vitro antibacterial activity was determined as inhibitory concentration causing 50% DNA-gyrase supercoiling inhibition (SCI)More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of xanthine oxidase assessed as decrease in uric acid production by spectrophotometryMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Dongguk University-Seoul
Curated by ChEMBL
Dongguk University-Seoul
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant FLT3 (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataKd: 5.89E+3nMAssay Description:Binding affinity to ABCB1 nucleotide binding domain 2More data for this Ligand-Target Pair
Target3-oxoacyl-acyl-carrier protein reductase(Plasmodium falciparum)
University of Zurich
Curated by ChEMBL
University of Zurich
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of FabGMore data for this Ligand-Target Pair
TargetCytochrome P450 1B1(Homo sapiens (Human))
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of human CYP1B1 by EROD assayMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of human CYP1A2 by EROD assayMore data for this Ligand-Target Pair
Affinity DataIC50: 77nMAssay Description:Inhibition of human CYP1A1 by EROD assayMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Kyoto Pharmaceutical University
Curated by ChEMBL
Kyoto Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of mushroom tyrosinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+5nMAssay Description:Inhibition of wild type Amyloid beta (1 to 42) (unknown origin) aggregation by Thioflavin-T fluorescence assayMore data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Mus musculus (Mouse))
Département de Pharmacochimie Moléculaire UMR-CNRS 5063
Curated by ChEMBL
Département de Pharmacochimie Moléculaire UMR-CNRS 5063
Curated by ChEMBL
Affinity DataKd: 5.90E+3nMAssay Description:Binding affinity of the compound to nucleotide-binding domain (NBD2) of P-GlycoproteinMore data for this Ligand-Target Pair
Affinity DataIC50: 2.96E+5nMAssay Description:Inhibition of p56 lckMore data for this Ligand-Target Pair
Affinity DataIC50: 2.34E+4nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+5nMpH: 7.4 T: 2°CAssay Description:Fluorescence intensity was measured at 420 nm excitation and 485 nm emission using a microplate reader (MPR-A4ιII; TOSOH, Tokyo, Japan, or Fluoroska...More data for this Ligand-Target Pair
TargetMitochondrial import inner membrane translocase subunit TIM10(Saccharomyces cerevisiae S288c)
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.00E+5nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA...More data for this Ligand-Target Pair
Affinity DataIC50: 9.80E+3nMAssay Description:The kinase assay was performed using the EMD Millipore KinaseProfiler service assay protocol. Aurora B kinase was supplied by EMD Millipore Corp. The...More data for this Ligand-Target Pair