null
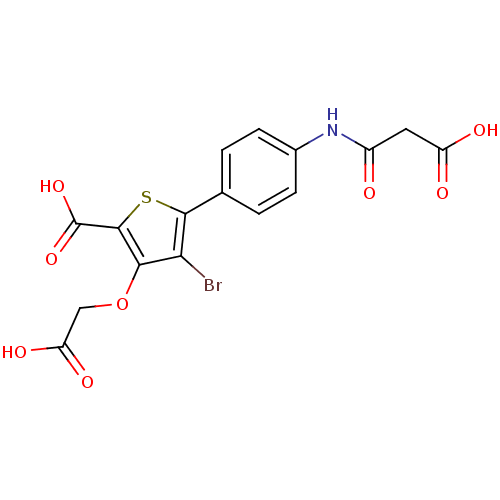
SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1ccc(NC(=O)CC(O)=O)cc1
InChI Key InChIKey=UYGDTHQGIZMLJM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 14267
Found 6 hits for monomerid = 14267
Affinity DataKi: 140nM ΔG°: -9.34kcal/moleT: 2°CAssay Description:The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: 180nM ΔG°: -9.19kcal/moleT: 2°CAssay Description:The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase C(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: 3.30E+4nMAssay Description:Inhibition of PTPRCMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase F(Homo sapiens (Human))
University of Miami
Curated by ChEMBL
University of Miami
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of PTPRFMore data for this Ligand-Target Pair