null
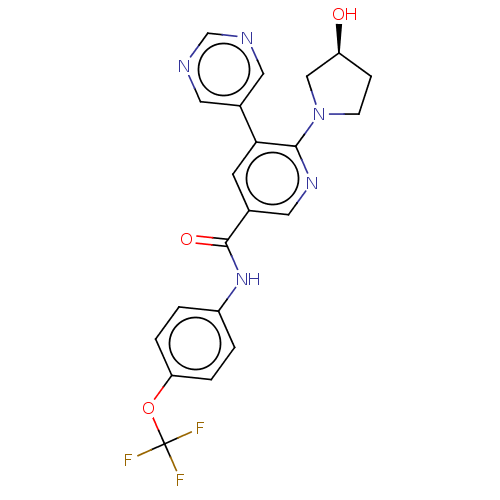
SMILES O[C@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1
InChI Key InChIKey=LARFZNXVNANWFD-INIZCTEOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 213441
Found 7 hits for monomerid = 213441
Affinity DataIC50: 9nMpH: 7.5 T: 2°CAssay Description:For determination of ABL kinase activity, the radiometric filter-binding assay was used. The assay was performed by mixing 10 uL of the compound pre-...More data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMpH: 7.5 T: 2°CAssay Description:The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of human ERG by manual patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 4.20E+3nMAssay Description:Inhibition of human ERG by automated patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 9.60E+3nMAssay Description:Displacement of [3H]dofetilide from human ERG by high throughput assayMore data for this Ligand-Target Pair