null
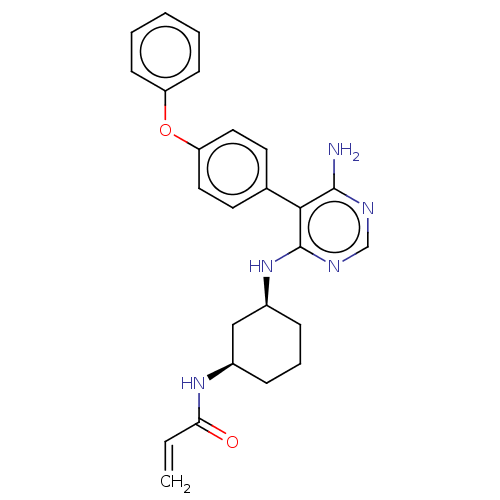
SMILES Nc1ncnc(N[C@H]2CCC[C@H](C2)NC(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1
InChI Key InChIKey=WFULWSGYPNUKDK-MOPGFXCFSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 291452
Found 6 hits for monomerid = 291452
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck KGaA
Curated by ChEMBL
Merck KGaA
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMpH: 7.5 T: 2°CAssay Description:The following describes a microfluidic, off-chip mobility shift kinase assay used to measure inherent potency of compounds against BTK enzyme. Compou...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:2.5× stocks of full-length human BTK (08-080) from CarnaBio USA, Inc., Natick, Mass., 1.6×ATP and appropriate kinKDR peptide substrate (FITC-AHA-EEPL...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMpH: 7.5 T: 2°CAssay Description:The following describes a microfluidic, off-chip mobility shift kinase assay used to measure inherent potency of compounds against BTK enzyme. Compou...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:2.5× stocks of full-length human BTK (08-080) from CarnaBio USA, Inc., Natick, Mass., 1.6×ATP and appropriate kinKDR peptide substrate (FITC-AHA-EEPL...More data for this Ligand-Target Pair