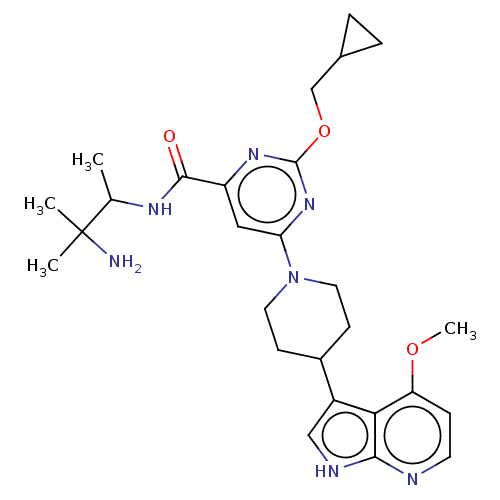
null
SMILES COc1ccnc2[nH]cc(C3CCN(CC3)c3cc(nc(OCC4CC4)n3)C(=O)NC(C)C(C)(C)N)c12
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 300077
Found 2 hits for monomerid = 300077
Affinity DataKi: <0.0130nMAssay Description:AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility...More data for this Ligand-Target Pair
Affinity DataKi: 0.75nMAssay Description:AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility...More data for this Ligand-Target Pair