null
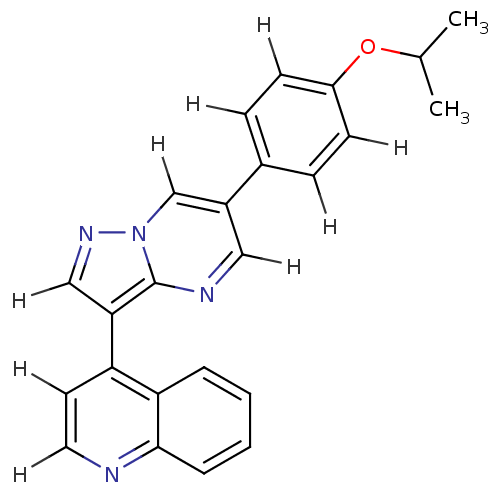
SMILES CC(C)Oc1ccc(cc1)-c1cnc2c(cnn2c1)-c1ccnc2ccccc12
InChI Key InChIKey=JMIFGARJSWXZSH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 36354
Found 15 hits for monomerid = 36354
Affinity DataEC50: 200nMAssay Description:The effects on zebrafish embryos with respect to the dorsoventral (DV) axis. For dorsalization, the EC100 (effective concentration 100%) represents t...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human ALK2 in presence of (33P)gamma ATPMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:For nonspecific toxicity, the EC100 represents the concentration when 100% of the treated embryos exhibit either early lethality within hours of comp...More data for this Ligand-Target Pair
Affinity DataIC50: 108nMAssay Description:Shown are the IC50s (concentrations causing 50% inhibition) of DM and the analogues for the in vitro kinase assays using the following purified human...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase receptor R3(Mus musculus)
Massachusetts Institute of Technology
Massachusetts Institute of Technology
TargetBone morphogenetic protein receptor type-1A(Mus musculus)
Massachusetts Institute of Technology
Massachusetts Institute of Technology
Affinity DataIC50: 108nMAssay Description:Kinase assays were performed using the assay kit by Reaction Biology Corp (Malvern, Pa.). The compounds were tested at 10 concentrations by 3-fold se...More data for this Ligand-Target Pair
TargetBone morphogenetic protein receptor type-1B(Homo sapiens (Human))
Vanderbilt University Medical Center
Curated by ChEMBL
Vanderbilt University Medical Center
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Inhibition of ALK6 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase receptor R3(Homo sapiens (Human))
Vanderbilt University Medical Center
Curated by ChEMBL
Vanderbilt University Medical Center
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Inhibition of ALK1 (unknown origin)More data for this Ligand-Target Pair
TargetActivin receptor type-1B(Homo sapiens (Human))
Vanderbilt University Medical Center
Curated by ChEMBL
Vanderbilt University Medical Center
Curated by ChEMBL
Affinity DataIC50: 9.62E+3nMAssay Description:Inhibition of ALK4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 108nMAssay Description:Inhibition of ALK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:For ISV disruption, the EC50 represents the concentration when the formation of about 50% of the ISVs is inhibited.More data for this Ligand-Target Pair