null
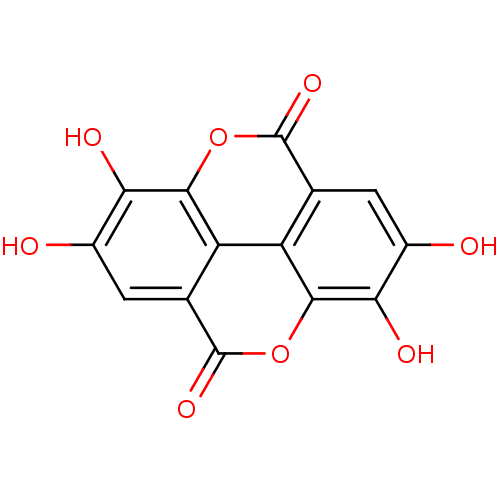
SMILES Oc1cc2c3c(oc(=O)c4cc(O)c(O)c(oc2=O)c34)c1O
InChI Key InChIKey=AFSDNFLWKVMVRB-UHFFFAOYSA-N
PDB links: 3 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 118 hits for monomerid = 4078
Found 118 hits for monomerid = 4078
TargetCasein kinase II subunit alpha(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Inhibition of CK2alpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.95E+3nMAssay Description:Inhibition of recombinant human MIF expressed in Escherichia coli BL21(DE3) using dopa as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 2.18E+3nMAssay Description:Inhibition of human CA2 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.32E+3nMAssay Description:Inhibition of human CA1 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Catholic University of Daegu
Catholic University of Daegu
Affinity DataKi: 2.80E+3nMAssay Description:The reaction mixtures contained in various different concentrations of p-NPP as a PTP1B substrate in the presence or absence of the active compound. ...More data for this Ligand-Target Pair
Affinity DataKi: 4.24E+3nMAssay Description:Inhibition of human carbonic anhydrase 13 preincubated for 6 hrs by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.53E+3nM IC50: 1.45E+4nMpH: 7.0Assay Description:Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c...More data for this Ligand-Target Pair
Affinity DataKi: 6.32E+3nMAssay Description:Inhibition of human CA7 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.06E+3nMAssay Description:Inhibition of human CA6 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
TargetCarbonic anhydrase 5A, mitochondrial(Homo sapiens (Human))
Universit£ degli Studi di Firenze
Curated by ChEMBL
Universit£ degli Studi di Firenze
Curated by ChEMBL
Affinity DataKi: 7.59E+3nMAssay Description:Inhibition of human CA5A by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.15E+3nMAssay Description:Inhibition of human carbonic anhydrase 12 preincubated for 6 hrs by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.79E+3nMAssay Description:Inhibition of human carbonic anhydrase 7 preincubated for 6 hrs by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.91E+3nMAssay Description:Inhibition of human CA14 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 9.08E+3nMAssay Description:Inhibition of human CA4 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 9.37E+3nMAssay Description:Inhibition of human CA9 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.01E+4nMAssay Description:Inhibition of human CA12 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.03E+4nMAssay Description:Inhibition of mouse CA13 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.05E+4nMAssay Description:Inhibition of human CA3 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
TargetCarbonic anhydrase 5B, mitochondrial(Homo sapiens (Human))
Universit£ degli Studi di Firenze
Curated by ChEMBL
Universit£ degli Studi di Firenze
Curated by ChEMBL
Affinity DataKi: 1.27E+4nMAssay Description:Inhibition of human CA5B by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.82E+4nMAssay Description:Inhibition of human carbonic anhydrase 1 preincubated for 6 hrs by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.98E+4nMAssay Description:Inhibition of human carbonic anhydrase 9 preincubated for 6 hrs by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of human carbonic anhydrase 2 preincubated for 6 hrs by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.46E+5nM ΔG°: -5.23kcal/molepH: 7.4 T: 2°CAssay Description:Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion using s...More data for this Ligand-Target Pair
Affinity DataKi: 2.07E+5nM ΔG°: -5.02kcal/molepH: 7.4 T: 2°CAssay Description:Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion using s...More data for this Ligand-Target Pair
Affinity DataIC50: 300nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration, which inhibits 50% of pp60c-src activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] ...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration, which inhibits 50% of PKA activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] label...More data for this Ligand-Target Pair
TargetHeat shock 70 kDa protein 1A(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 760nMpH: 7.0 T: 2°CAssay Description:Burnham Center for Chemical Genomics (BCCG) Burnham Institute for Medical Research (San Diego, CA) NIH Molecular Libraries Screening Centers Network ...More data for this Ligand-Target Pair
TargetHeat shock 70 kDa protein 1A(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.00E+5nMAssay Description:Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (San Diego, CA) NIH Molecular Libraries Screening Cen...More data for this Ligand-Target Pair
TargetAcetyl-CoA acetyltransferase/HMG-CoA reductase(Enterococcus faecalis)
SRMLSC
Curated by PubChem BioAssay
SRMLSC
Curated by PubChem BioAssay
Affinity DataIC50: 1.27E+6nMAssay Description:Southern Research Molecular Libraries Screening Center (SRMLSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Screening C...More data for this Ligand-Target Pair
TargetMothers against decapentaplegic homolog 3(Homo sapiens (Human))
Emory University Molecular Libraries Screening Center
Curated by PubChem BioAssay
Emory University Molecular Libraries Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 142nMAssay Description:NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: F.M. Hoffmann, University ...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 4 gamma 1(Homo sapiens (Human))
Emory University Molecular Libraries Screening Center
Curated by PubChem BioAssay
Emory University Molecular Libraries Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 4.77E+4nMAssay Description:Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation NIH Molecular Libraries Screening Centers Network [MLSC...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1(Homo sapiens (Human))
Emory University Molecular Libraries Screening Center
Curated by PubChem BioAssay
Emory University Molecular Libraries Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 5.40E+4nMAssay Description:NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: Nikolovska-Coleska, Univer...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 6(Rattus norvegicus)
Sanford-Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Sanford-Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 3.95E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetHeat shock cognate 71 kDa protein(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 9.92E+4nMAssay Description:Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (San Diego, CA) NIH Molecular Libraries Screening Cen...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.23E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Target2'-phosphotransferase(Candida albicans SC5314)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 541nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS...More data for this Ligand-Target Pair
TargetGuanyl-specific ribonuclease T1(Aspergillus oryzae)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 5.57E+4nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS...More data for this Ligand-Target Pair
Affinity DataIC50: 1.38E+5nMAssay Description:Southern Research Molecular Libraries Screening Center (SRMLSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Screening...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 980nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.8Assay Description:The reaction mixture contained DNA (1.25 uM or as specified), NTP (110 uM or as specified), 50 mM NaCl, 150 mM potassium glutamate, buffer [20 mM CAP...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:A range of natural products were screened for inhibition of PfGST by GST assay with CDNB as a substrate, using a 96-well SpectraMax 340 microplate sp...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Catholic University of Daegu
Catholic University of Daegu
Affinity DataIC50: 7.73E+3nMpH: 6.0Assay Description:PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.0 T: 2°CAssay Description:Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+5nMAssay Description:Inhibition of cruzain in presence of 0.01% Triton X-100More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 2.07E+4nMAssay Description:Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
TargetcAMP-dependent protein kinase catalytic subunit alpha/beta/gamma(Homo sapiens (Human))
Università di Padova
Curated by ChEMBL
Università di Padova
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of PKAMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor/Nucleophosmin(Homo sapiens (Human))
Università di Padova
Curated by ChEMBL
Università di Padova
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of NPM-ALKMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Westf£lische Wilhelms-Universit£t M£nster
Curated by ChEMBL
Westf£lische Wilhelms-Universit£t M£nster
Curated by ChEMBL
Affinity DataIC50: 3.39E+3nMAssay Description:Inhibition of human recombinant CDK2/CyclinA expressed in Sf9 cells using histone H1 as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
TargetAurora kinase B(Homo sapiens (Human))
Westf£lische Wilhelms-Universit£t M£nster
Curated by ChEMBL
Westf£lische Wilhelms-Universit£t M£nster
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human recombinant aurora-B expressed in Sf9 cells using tetra(LRRWSLG) as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)