null
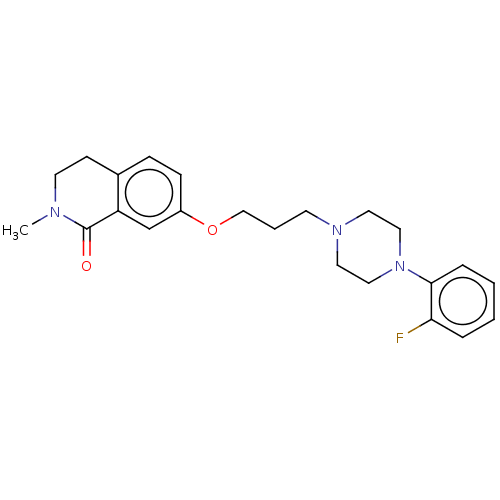
SMILES CN1CCc2ccc(OCCCN3CCN(CC3)c3ccccc3F)cc2C1=O
InChI Key InChIKey=JSYJUUGAOMCCSL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 423314
Found 8 hits for monomerid = 423314
Affinity DataKi: 24.5nMAssay Description:5-HT1A: (1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed ...More data for this Ligand-Target Pair
Affinity DataKi: 29.9nMAssay Description:5-HT7: (1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container...More data for this Ligand-Target Pair
Affinity DataKi: 32.1nMAssay Description:5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container...More data for this Ligand-Target Pair
Affinity DataKi: 54.8nMAssay Description:D2:Procedures(1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were m...More data for this Ligand-Target Pair
Affinity DataKi: 117nMAssay Description:Displacement of [3H]spiperone from D2 receptor in rat striatum measured after 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 262nMAssay Description:Displacement of [3H](+)8-OH-DPAT from rat cerebral cortex 5HT1A receptor measured after 30 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 306nMAssay Description:Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Histamine H1: (1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were ...More data for this Ligand-Target Pair