null
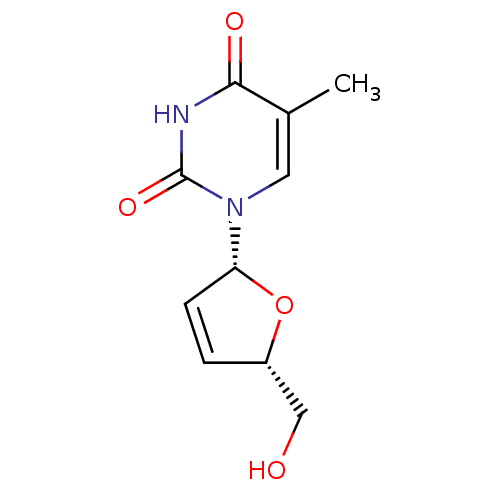
SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O
InChI Key InChIKey=XNKLLVCARDGLGL-JGVFFNPUSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 50013111
Found 17 hits for monomerid = 50013111
Affinity DataKi: 5.80E+5nMAssay Description:Inhibitory affect against rabbit thymus thymidine kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 6.15E+5nMAssay Description:Inhibitory affect against rabbit thymus thymidine kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+6nMAssay Description:Competitive inhibition against rat cytoplasmic Thymidine kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.25E+6nMAssay Description:Competitive inhibition against rat mitochondrial thymidine kinaseMore data for this Ligand-Target Pair
TargetReverse transcriptase/RNaseH(Human immunodeficiency virus 1)
The University of Georgia
Curated by ChEMBL
The University of Georgia
Curated by ChEMBL
Affinity DataEC50: 450nMAssay Description:In vitro antiviral activity against HIV-1 Reverse transcriptase wild typeMore data for this Ligand-Target Pair
TargetReverse transcriptase/RNaseH(Human immunodeficiency virus 1)
The University of Georgia
Curated by ChEMBL
The University of Georgia
Curated by ChEMBL
Affinity DataEC50: 50nMAssay Description:Effective concentration that reduces HIV-induced cytopathic effect by 50% was determined by reverse transcriptase (RT) assay.More data for this Ligand-Target Pair
TargetReverse transcriptase/RNaseH(Human immunodeficiency virus 1)
The University of Georgia
Curated by ChEMBL
The University of Georgia
Curated by ChEMBL
Affinity DataEC50: 500nMAssay Description:In vitro antiviral activity against HIV-1 Reverse transcriptase M184V mutantMore data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
Affinity DataKd: 4.40E+4nMAssay Description:Binding affinity to human serum albumin with excitation at 280 nm after 2 hrs by spectrofluorimetric analysisMore data for this Ligand-Target Pair
TargetRecBCD enzyme subunit RecD(Escherichia coli str. K-12 substr. MG1655)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 9.60E+3nMAssay Description:Inhibition of HIV1 reverse transcriptase-mediated thymidine incorporation into D23/D36 primer-template preincubated for 15 mins by polyacrylamide gel...More data for this Ligand-Target Pair
TargetRecBCD enzyme subunit RecD(Escherichia coli str. K-12 substr. MG1655)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
TargetRecBCD enzyme subunit RecD(Escherichia coli str. K-12 substr. MG1655)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay