null
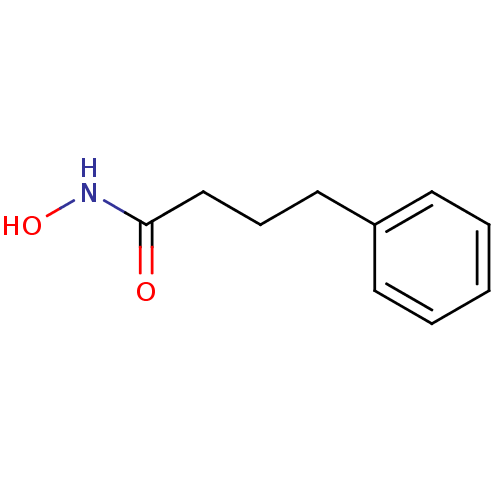
SMILES ONC(=O)CCCc1ccccc1
InChI Key InChIKey=UPHXPXYRKPCXHK-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 50015142
Found 13 hits for monomerid = 50015142
TargetPolyunsaturated fatty acid lipoxygenase ALOX15(Rattus norvegicus)
Rorer Central Research
Curated by ChEMBL
Rorer Central Research
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Invitro inhibition of rat platelet 12-lipoxygenaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+4nMAssay Description:In vitro inhibitory activity against RBL-1 5-LOMore data for this Ligand-Target Pair
Affinity DataIC50: 2.69E+4nMAssay Description:Logarithmic value of inhibitory concentration against 5-lipoxygenase in rat basophilic leukemia cells (RBL-1)More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+4nMAssay Description:In vitro inhibitory activity against 5-lipoxygenase in rat basophilic leukemia cells(RBL-1)More data for this Ligand-Target Pair
TargetHistone deacetylase 1(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of human recombinant HDAC1More data for this Ligand-Target Pair
TargetHistone deacetylase 2(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of human recombinant HDAC2More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibition of human recombinant HDAC6More data for this Ligand-Target Pair
TargetHistone deacetylase 8(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human recombinant HDAC8More data for this Ligand-Target Pair
TargetHistone deacetylase 4(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 1.40E+5nMAssay Description:Inhibition of human recombinant HDAC4More data for this Ligand-Target Pair
TargetHistone deacetylase 5(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human recombinant HDAC5More data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 1.50E+5nMAssay Description:Inhibition of human recombinant HDAC7More data for this Ligand-Target Pair
TargetHistone deacetylase 9(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 4.30E+5nMAssay Description:Inhibition of human recombinant HDAC9More data for this Ligand-Target Pair
TargetHistone deacetylase 3(Homo sapiens (Human))
Broad Institute of Harvard and MIT
Curated by ChEMBL
Broad Institute of Harvard and MIT
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human recombinant HDAC3More data for this Ligand-Target Pair