null
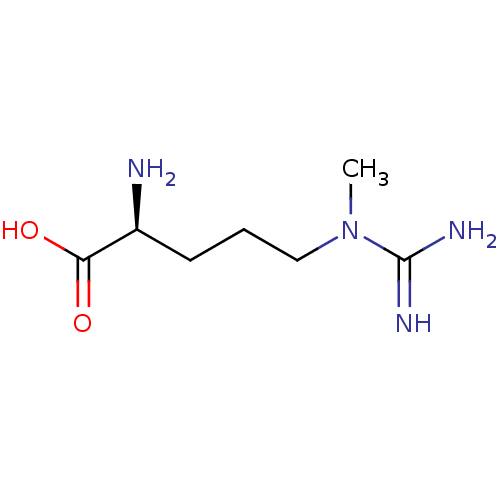
SMILES CN(CCC[C@H](N)C(O)=O)C(N)=N
InChI Key InChIKey=XKCWNEVAXQCMGP-YFKPBYRVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50049254
Found 9 hits for monomerid = 50049254
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 1.40E+6nMAssay Description:Binding affinity towards inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibitory activity against human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research and Development
Curated by ChEMBL
G. D. Searle Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity against human Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research and Development
Curated by ChEMBL
G. D. Searle Research and Development
Curated by ChEMBL
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibitory activity against human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataIC50: 1.00E+7nMAssay Description:Inhibition of human recombinant iNOS by griess assayMore data for this Ligand-Target Pair
TargetN(G),N(G)-dimethylarginine dimethylaminohydrolase 1(Homo sapiens (Human))
Christian-Albrechts-University of Kiel
Curated by ChEMBL
Christian-Albrechts-University of Kiel
Curated by ChEMBL
Affinity DataIC50: 5.00E+6nMAssay Description:Inhibition of human recombinant DDAH1 expressed in Escherichia coli BL21 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research and Development
Curated by ChEMBL
G. D. Searle Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+7nMAssay Description:Inhibition of human recombinant eNOS by griess assayMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research and Development
Curated by ChEMBL
G. D. Searle Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+7nMAssay Description:Inhibition of human recombinant nNOS by griess assayMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
Université de Haute-Alsace
Curated by ChEMBL
Université de Haute-Alsace
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Compound was evaluated for inhibitory activity against iNOS obtained from RAW 264.7 cells.More data for this Ligand-Target Pair