null
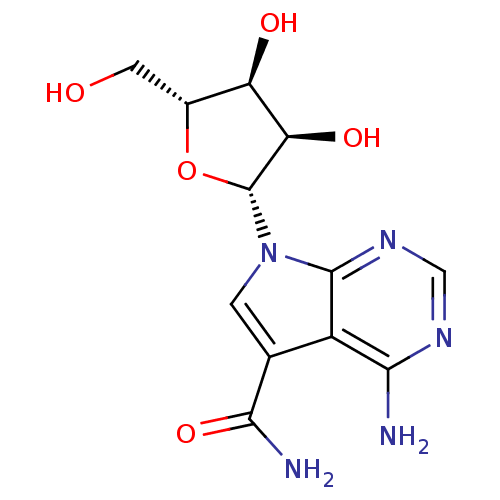
SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12
InChI Key InChIKey=OBZJZDHRXBKKTJ-JTFADIMSSA-N
PDB links: 12 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50049820
Found 11 hits for monomerid = 50049820
TargetRhodopsin kinase GRK1(Homo sapiens (Human))
Georgetown University Medical Center
Curated by ChEMBL
Georgetown University Medical Center
Curated by ChEMBL
Affinity DataKi: 180nMAssay Description:Inhibition of rhodopsin kinase (unknown origin)More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Georgetown University Medical Center
Curated by ChEMBL
Georgetown University Medical Center
Curated by ChEMBL
Affinity DataKi: 6.70E+4nMAssay Description:Inhibition of beta-adrenergic receptor kinase(unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 470nMAssay Description:Inhibition of recombinant human adenosine kinaseMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 2.60E+4nMAssay Description:Inhibition of DOT1L (unknown origin) using chicken nucleosome as substrate in presence of [3H]SAM incubated for 1 hr by TopCount methodMore data for this Ligand-Target Pair
TargetHeat shock cognate 71 kDa protein(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataKd: 3.30E+3nMAssay Description:Binding affinity to human truncated HSC70 NBD (1 to 381 residues) by SPR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 467nMAssay Description:Concentration required for 50% inhibition of the adenosine kinase (AK) activity.More data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+8nMAssay Description:Inhibition of adenosine kinase (unknown origin)More data for this Ligand-Target Pair
TargetAdenosine kinase(Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
Texas A&M University
Curated by ChEMBL
Texas A&M University
Curated by ChEMBL
Affinity DataIC50: 1.65E+4nMAssay Description:Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli...More data for this Ligand-Target Pair
Affinity DataIC50: 470nMAssay Description:Inhibition of human adenosine kinase activityMore data for this Ligand-Target Pair
TargetGalanin receptor type 3(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
TargetHeat shock cognate 71 kDa protein(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataKd: 3.24E+3nMAssay Description:Binding affinity to human truncated HSC70 NBD (1 to 381 residues) by SPR analysisMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)