null
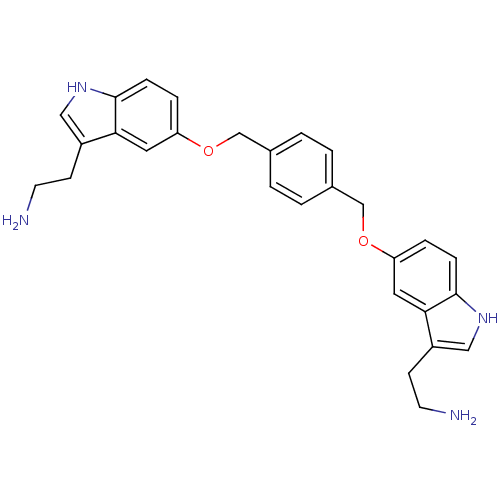
SMILES NCCc1c[nH]c2ccc(OCc3ccc(COc4ccc5[nH]cc(CCN)c5c4)cc3)cc12
InChI Key InChIKey=IFTLFEAARDEHJJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50054974
Found 7 hits for monomerid = 50054974
Affinity DataKi: 0.100nMAssay Description:Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Centre de Recherche Pierre Fabre
Curated by ChEMBL
Centre de Recherche Pierre Fabre
Curated by ChEMBL
Affinity DataKi: 0.110nMAssay Description:Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
Centre de Recherche Pierre Fabre
Curated by ChEMBL
Centre de Recherche Pierre Fabre
Curated by ChEMBL
Affinity DataKi: 0.110nMAssay Description:Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Binding affinity to 5HT1A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1A receptor in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 3nMAssay Description:Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair