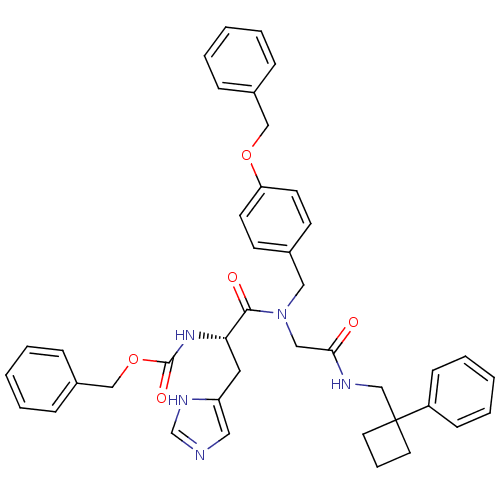
null
SMILES O=C(CN(Cc1ccc(OCc2ccccc2)cc1)C(=O)[C@H](Cc1cnc[nH]1)NC(=O)OCc1ccccc1)NCC1(CCC1)c1ccccc1
InChI Key InChIKey=HNXRTWHXWCHGID-QNGWXLTQSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50060139
Found 2 hits for monomerid = 50060139
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Rattus norvegicus)
Warner-Lambert Company
Curated by ChEMBL
Warner-Lambert Company
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:In vitro inhibition of rat farnesyltransferase.More data for this Ligand-Target Pair
Affinity DataIC50: 88nMAssay Description:Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells .More data for this Ligand-Target Pair