null
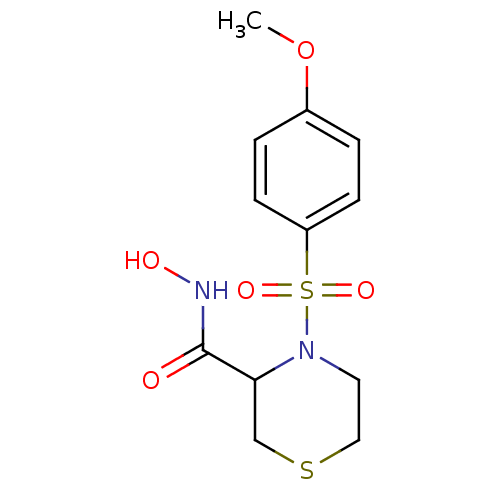
SMILES COc1ccc(cc1)S(=O)(=O)N1CCSCC1C(=O)NO
InChI Key InChIKey=SKVZRQFKXKDXCW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50082555
Found 8 hits for monomerid = 50082555
Affinity DataIC50: 15nMAssay Description:In vitro inhibitory activity against matrix metalloprotease-3.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In vitro inhibitory activity against collagenase-3 (matrix metalloprotease-13).More data for this Ligand-Target Pair
Affinity DataIC50: 2.07E+3nMAssay Description:In vitro inhibitory activity against matrilysin (matrix metalloprotease-7).More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of truncated human recombinant MMP3 catalytic domain expressed in Escherichia coli BL21(DE3) after 3 hrs in presence of [H]-transferrinMore data for this Ligand-Target Pair
Affinity DataIC50: 2.03E+3nMAssay Description:Inhibition of MMP7 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 min...More data for this Ligand-Target Pair
TargetInterstitial collagenase(Human)
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States.
Curated by ChEMBL
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States.
Curated by ChEMBL
Affinity DataIC50: 62nMAssay Description:Inhibition of human interstitial recombinant N-terminal MMP1 expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as sub...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of MMP13 (unknown origin) expressed in Escherichia coli BL21(DE3) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate after 20 to 30 mi...More data for this Ligand-Target Pair
TargetInterstitial collagenase(Human)
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States.
Curated by ChEMBL
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States.
Curated by ChEMBL
Affinity DataIC50: 62nMAssay Description:In vitro inhibitory activity against truncated collagenase-1 (matrix metalloprotease-1).More data for this Ligand-Target Pair