null
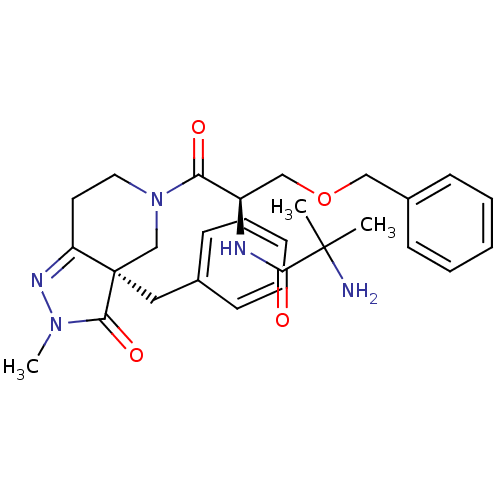
SMILES CN1N=C2CCN(C[C@@]2(Cc2ccccc2)C1=O)C(=O)[C@@H](COCc1ccccc1)NC(=O)C(C)(C)N
InChI Key InChIKey=KVLLHLWBPNCVNR-SKCUWOTOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50083974
Found 7 hits for monomerid = 50083974
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ...More data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataEC50: 0.912nMAssay Description:In vitro inhibition of dihydrofolate reductase enzymes in Neisseria gonorrhoeaeMore data for this Ligand-Target Pair
Affinity DataEC50: 3nMAssay Description:Agonist activity at GHSR in Wistar rat pituitary gland cells assessed as induction of GH release by RIAMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataEC50: 0.900nMAssay Description:Ability to inhibit HMG-CoA reductase (HMGR) by cholesterol synthesis inhibition screen (CSI) in ratsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Displacement of human [125I]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes after 1 hr by radioligand binding assayMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Binding affinity for human growth hormone GH secretagogue (hGHsr) receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:In vitro inhibition of dihydrofolate reductase enzymes in Neisseria gonorrhoeaeMore data for this Ligand-Target Pair