null
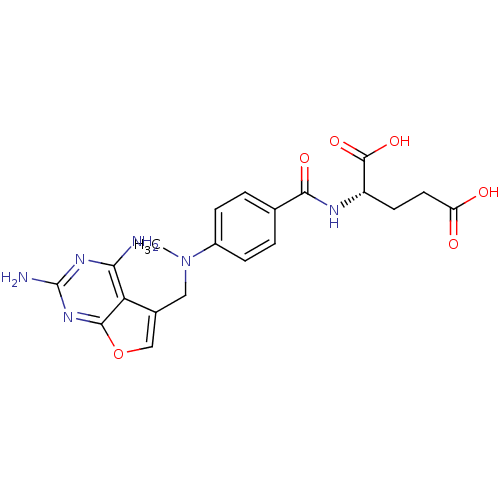
SMILES CN(Cc1coc2nc(N)nc(N)c12)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O
InChI Key InChIKey=WXINNGCGSCFUCR-ZDUSSCGKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50091145
Found 9 hits for monomerid = 50091145
Affinity DataIC50: 220nMAssay Description:Inhibitory concentration against Lactobacillus casei Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibitory concentration against recombinant human (rh) Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.98E+4nMAssay Description:Inhibitory concentration against Toxoplasma gondii Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibitory concentration against recombinant human (rh) Thymidylate synthase (TS)More data for this Ligand-Target Pair
Affinity DataIC50: 220nMpH: 7.4Assay Description:Inhibition of human DHFR at 30 degC under pH 7.4 by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibitory concentration against human Thymidylate synthase(TS)More data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibitory concentration against human dihydrofolate reductase (DHFR)More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMpH: 7.4Assay Description:Inhibition of human thymidylate synthase at 30 degC under pH 7.4 by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibitory concentration against Lactobacillus casei Thymidylate synthase (TS).More data for this Ligand-Target Pair