null
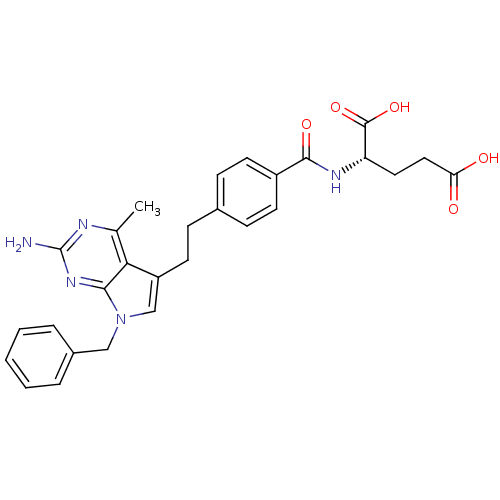
SMILES Cc1nc(N)nc2n(Cc3ccccc3)cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c12
InChI Key InChIKey=XYVUDAYNSCLZJK-QFIPXVFZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50092652
Found 9 hits for monomerid = 50092652
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibitory concentration against isolated human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibitory concentration against isolated Escherichia coli Thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibitory concentration against isolated rat thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibitory concentration against isolated Thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibitory concentration against isolated Pneumocystis carinii DHFR (Dihydrofolate reductase)More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibitory concentration against isolated Escherichia coli Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibitory concentration against isolated Lactobacillus casei Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 310nMAssay Description:Inhibitory concentration against isolated Lactobacillus casei Thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibitory concentration against isolated Toxoplasma gondii DHFR (dihydrofolate reductase)More data for this Ligand-Target Pair