null
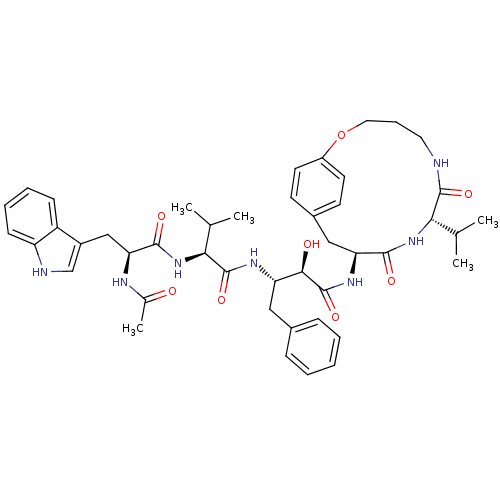
SMILES CC(C)[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)N[C@H]1Cc2ccc(OCCCNC(=O)[C@@H](NC1=O)C(C)C)cc2
InChI Key InChIKey=NEFLBYBCIDVAIQ-BYAJYZPISA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50096445
Found 9 hits for monomerid = 50096445
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Inhibitory concentration of the compound against TL3-resistant HIV(M461) mutant proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Inhibitory concentration of the compound against drug-resistant HIV(G48V) mutant proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 71nMAssay Description:Inhibitory concentration of the compound against TL3-resistant HIV(V771) mutant proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibitory concentration of the compound against TL3-resistant HIV(V82A) mutantMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibitory concentration of the compound against TL3-resistant HIV(V771) mutantMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibitory concentration of the compound against TL3-resistant HIV(L241) mutant proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Inhibitory concentration of the compound against TL3-resistant HIV(L241) mutant proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Inhibitory concentration of the compound against TL3-resistant HIV(V771) mutant proteaseMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Inhibitory concentration of the compound against TL3-resistant HIV(F53L) mutant proteaseMore data for this Ligand-Target Pair