null
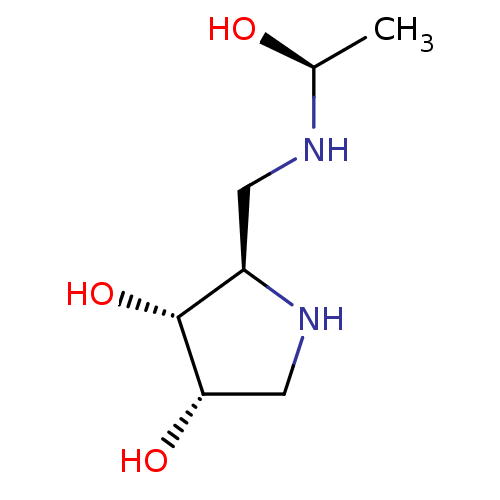
SMILES C[C@H](O)NC[C@H]1NC[C@H](O)[C@@H]1O
InChI Key InChIKey=ILPLXBQAXZPQTP-BJMCJPPVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50104301
Found 9 hits for monomerid = 50104301
Targetalpha-1,2-Mannosidase(Glycine max)
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Affinity DataKi: 1.20E+5nMAssay Description:Inhibitory activity towards Alpha-mannosidase from Jack beanMore data for this Ligand-Target Pair
TargetBeta-glucosidase A(Caldocellum saccharolyticum)
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Affinity DataKi: 1.70E+5nMAssay Description:Inhibitory activity towards Beta-Glucosidase from Caldocellum saccharol.More data for this Ligand-Target Pair
Affinity DataKi: 3.40E+5nMAssay Description:Inhibitory activity towards Beta-Glucosidase from Caldocellum saccharol.More data for this Ligand-Target Pair
TargetEndoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase(Homo sapiens (Human))
Universidade do Porto 687
Curated by ChEMBL
Universidade do Porto 687
Curated by ChEMBL
Affinity DataKi: 4.88E+5nMAssay Description:Inhibition of human ER alpha mannosidase 1More data for this Ligand-Target Pair
Affinity DataKi: 6.83E+5nMAssay Description:Inhibition of human golgi alpha mannosidase 2More data for this Ligand-Target Pair
TargetBeta-galactosidase(Aspergillus niger)
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Affinity DataIC50: 4.80E+5nMAssay Description:Inhibitory activity towards Beta-galactosidase from Aspergillus nigerMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+5nMAssay Description:Inhibitory activity towards Beta-Glucosidase from AlmondMore data for this Ligand-Target Pair
TargetBeta-glucosidase A(Caldocellum saccharolyticum)
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Affinity DataIC50: 1.80E+5nMAssay Description:Inhibitory activity towards Beta-Glucosidase from Caldocellum saccharol.More data for this Ligand-Target Pair
Targetalpha-1,2-Mannosidase(Glycine max)
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Affinity DataIC50: 4.90E+5nMAssay Description:Inhibitory activity towards Alpha-mannosidase from Jack beanMore data for this Ligand-Target Pair