null
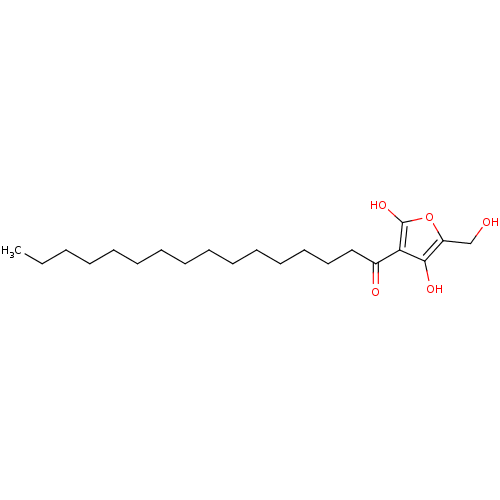
SMILES CCCCCCCCCCCCCCCC(=O)c1c(O)oc(CO)c1O
InChI Key InChIKey=BKBKOAWPAIKUCJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 27 hits for monomerid = 50104694
Found 27 hits for monomerid = 50104694
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibitory activity against cell division cycle 25BMore data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:Inhibition of human recombinant PTP1B assessed as p-nitorphenol production after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.16E+4nMAssay Description:Inhibitory activity against vaccinia VH1-related phosphatase(VHR)More data for this Ligand-Target Pair
Affinity DataIC50: 1.17E+4nMAssay Description:Inhibitory activity against vaccinia VH1-related phosphatase(VHR)More data for this Ligand-Target Pair
Affinity DataIC50: 4.49E+3nMAssay Description:Inhibition of PTP1B (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+4nMAssay Description:Inhibition of VHR DS-PTPMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibition of 6-His-N-terminal tagged human PTP1B expressed in Escherichia coli BL21 (DE3) cells assessed as pNP formation using pNPP as substrate in...More data for this Ligand-Target Pair
Affinity DataIC50: 1.24E+4nMAssay Description:Inhibition of 6-His-N-terminal tagged human CDC-25B isoform 3 catalytic domain expressed in Escherichia coli BL21 (DE3) cells assessed as pNP formati...More data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Human)
University of Campinas
Curated by ChEMBL
University of Campinas
Curated by ChEMBL
Affinity DataIC50: 8.60E+3nMAssay Description:Inhibition of full length 6-His-N-terminal tagged human LMW-PTP expressed in Escherichia coli BL21 (DE3) cells assessed as pNP formation using pNPP a...More data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+4nMAssay Description:Inhibition of PTP1B by colorimetric assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 4.60E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using p-nitrophenyl phosphate as substrateMore data for this Ligand-Target Pair
TargetHeparanase(Human)
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3)More data for this Ligand-Target Pair
Affinity DataIC50: 5.10E+3nMAssay Description:Inhibition of TCPTP (unknown origin) assessed as decrease in p-nitrophenol production from pNPP substrate after 30 mins by HTS7000 bioassay reader an...More data for this Ligand-Target Pair
Affinity DataIC50: 5.62E+3nMAssay Description:Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenol production from pNPP substrate after 30 mins by HTS7000 bioassay reader an...More data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of human recombinant PTP1B assessed as p-nitrophenol productionMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of human recombinant PTP1B after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate assessed as p-nitrophenol release after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.10E+3nMAssay Description:Inhibitory activity against cell division cycle 25BMore data for this Ligand-Target Pair