null
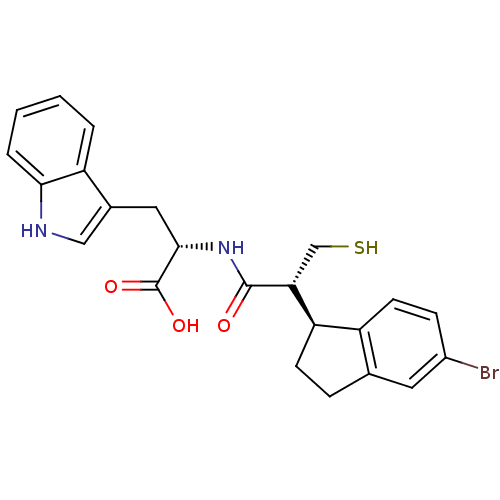
SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)[C@@H]1CCc2cc(Br)ccc12
InChI Key InChIKey=STCPANYJWPVHTG-SBHAEUEKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50115847
Found 3 hits for monomerid = 50115847
Affinity DataKi: 13nMAssay Description:In vitro inhibition of Neutral endopeptidase.More data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:In vitro inhibition of Angiotensin I converting enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 90nMAssay Description:In vitro inhibition of endothelin converting enzyme.More data for this Ligand-Target Pair