null
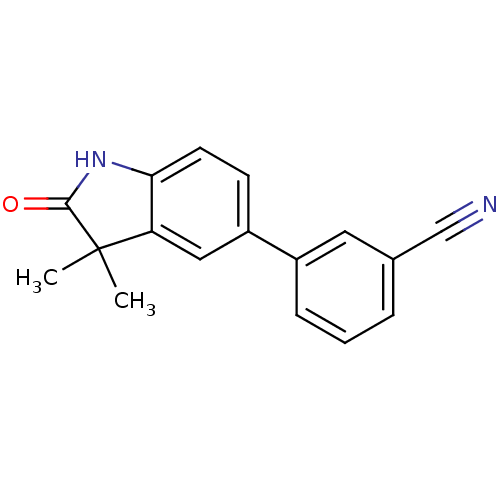
SMILES CC1(C)C(=O)Nc2ccc(cc12)-c1cccc(c1)C#N
InChI Key InChIKey=WZZPSUKZMZKXTQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50121164
Found 2 hits for monomerid = 50121164
Affinity DataIC50: 27nMAssay Description:Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphataseMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair