null
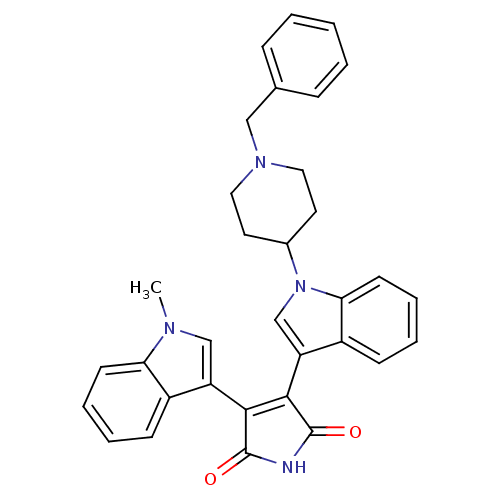
SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(Cc4ccccc4)CC3)c3ccccc23)c2ccccc12
InChI Key InChIKey=IKOMOLGHHLGWPZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50128284
Found 12 hits for monomerid = 50128284
Affinity DataIC50: 500nMAssay Description:Inhibition of Protein kinase C etaMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of Protein kinase C alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Protein kinase C zetaMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Src protein tryrosine kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of Protein kinase C beta 2More data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of purified mammalian brain [Ca(2+)]/Calmodulin dependent kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of Protein kinase C gammaMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of Protein kinase C deltaMore data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase type II subunit delta(Homo sapiens (Human))
Scios, Inc.
Curated by ChEMBL
Scios, Inc.
Curated by ChEMBL
Affinity DataIC50: 1.87E+3nMAssay Description:Inhibition of CaMK2deltaMore data for this Ligand-Target Pair
TargetProtein kinase C alpha/beta/delta/epsilon/eta/gamma/theta/zeta type(Rattus norvegicus)
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of purified rat brain protein kinase C (RB-PKC)More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of Protein kinase C beta 1More data for this Ligand-Target Pair
TargetProtein kinase C epsilon type(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of Protein kinase C epsilonMore data for this Ligand-Target Pair