null
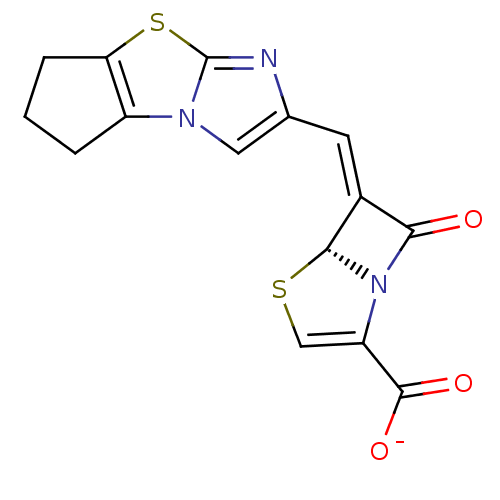
SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1
InChI Key InChIKey=XFARUGBBAMTOCU-IBSXUBTNSA-M
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50149467
Found 8 hits for monomerid = 50149467
Affinity DataIC50: 1nMAssay Description:In vitro inhibitory activity against Class A (TEM-1) beta-LactamasesMore data for this Ligand-Target Pair
Affinity DataIC50: 72nMAssay Description:In vitro inhibitory activity against Class A (Imi-1) beta-LactamasesMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:In vitro inhibitory activity against Class C (Amp-C) beta-LactamasesMore data for this Ligand-Target Pair
TargetBeta-lactamase SHV-1(Pseudomonas aeruginosa (g-Proteobacteria))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of Escherichia coli SHV1More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of Escherichia coli TEM1More data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:Inhibition of Bacteroides fragilis CcrAMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of Enterobacter cloacae AmpCMore data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:In vitro inhibitory activity against Class B (CCRA) beta-LactamasesMore data for this Ligand-Target Pair