null
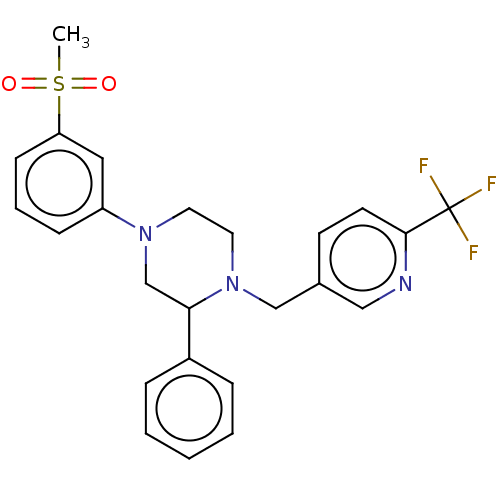
SMILES CS(=O)(=O)c1cccc(c1)N1CCN(Cc2ccc(nc2)C(F)(F)F)C(C1)c1ccccc1
InChI Key InChIKey=CTCGRQVHZGKQJF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50177013
Found 12 hits for monomerid = 50177013
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Affinity DataKi: 1.27E+3nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Affinity DataKi: 1.28E+3nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Affinity DataKi: 2.20E+3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Affinity DataKi: >2.50E+3nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Affinity DataKi: >3.30E+3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Affinity DataKi: >3.30E+3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Affinity DataEC50: 990nMAssay Description:Agonist activity at human LXRbeta ligand binding domain(155 to 460 residues) transfected in HEK293 cells after 16 hrs by Gal4-luciferase reporter gen...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Affinity DataEC50: 5.38E+3nMAssay Description:Agonist activity at human LXRalpha ligand binding domain(167 to 447 residues) transfected in HEK293 cells after 16 hrs by Gal4-luciferase reporter ge...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Affinity DataEC50: 5.37E+3nMAssay Description:Agonist activity at human LXRalpha ligand binding domain(167 to 447 residues) transfected in HEK293 cells after 16 hrs by Gal4-luciferase reporter ge...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Affinity DataEC50: >2.00E+4nMAssay Description:Agonist activity at human LXRalpha ligand binding domain(167 to 447 residues) transfected in HEK293 cells after 16 hrs by Gal4-luciferase reporter ge...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Affinity DataEC50: 2.14E+3nMAssay Description:Agonist activity at human LXRbeta ligand binding domain(155 to 460 residues) transfected in HEK293 cells after 16 hrs by Gal4-luciferase reporter gen...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
Affinity DataEC50: 2.13E+3nMAssay Description:Agonist activity at human LXRbeta ligand binding domain(155 to 460 residues) transfected in HEK293 cells after 16 hrs by Gal4-luciferase reporter gen...More data for this Ligand-Target Pair